Advanced pharmaceutical bulletin. 11(4):601-617.
doi: 10.34172/apb.2021.090
Review Article
Medicinal Plants in the Treatment of Hypertension: A Review
Raha Kamyab 1  , Hossein Namdar 2, Mohammadali Torbati 3, *
, Hossein Namdar 2, Mohammadali Torbati 3, *  , Morteza Ghojazadeh 4, Mostafa Araj-Khodaei 1, 5, Seyyed Mohammad Bagher Fazljou 1, *
, Morteza Ghojazadeh 4, Mostafa Araj-Khodaei 1, 5, Seyyed Mohammad Bagher Fazljou 1, * 
Author information:
1Department of Persian Medicine, Faculty of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
2Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3Department of Food Science and Technology, Faculty of Nutrition, Tabriz University of Medical Science, Tabriz, Iran.
4Research Center for Evidence Based Medicine (RCEBM), Tabriz University of Medical Sciences, Tabriz, Iran.
5Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
Traditional medicine is a comprehensive term for ancient, culture-bound health care practices that existed before the use of science in health matters and has been used for centuries. Medicinal plants are used to treat patients with cardiovascular diseases, which may occur due to ailments of the heart and blood vessels and comprise heart attacks, cerebrovascular diseases, hypertension, and heart failure. Hypertension causes difficulty in the functioning of the heart and is involved in atherosclerosis, raising the risk of heart attack and stroke. Many drugs are available for managing these diseases, though common antihypertensive drugs are generally accompanied by many side effects. Medicinal herbs have several active substances with pharmacological and prophylactic properties that can be used in the treatment of hypertension. This review presents an overview of some medicinal plants that have been shown to have hypotensive or antihypertensive properties.
Keywords: Traditional medicine, Hypertension management, Herbal medicine, Persian medicine, Cardiovascular diseases
Copyright and License Information
© 2021 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Cardiovascular diseases (CVDs) are a major cause of weakness and early death and, therefore, constitute a main communal health problem.
1
High blood pressure (BP), mentioned as a silent killer, is triggered by a range of factors, including the interaction of genetic and environmental components causing disorderliness in BP regulation.
2
Hypertension (HTN) is the most common risk factor in acute myocardial infarction and is accountable for about 16.5% deaths annually across the world. It is also the most important reason for the morbidity and mortality accompanying CVDs.
3
It has been predicted that by the year 2025, 29% of the world’s adults, or almost 1.56 billion people, will suffer from HTN.
4
HTN is described as systolic blood pressure (SBP) ≥ 140 mm Hg and diastolic blood pressure (DBP) ≥ 90 mm Hg, according to the mean of 2 or more appropriate measurements of seated BP.
5
Many antihypertensive mediators are used for the treatment of HTN, such as diuretics, sympatholytic agents, renin inhibitors, angiotensin converting enzyme (ACE) inhibitors, calcium channel blockers, β-adrenergic and α1/β-adrenergic antagonists, and vasodilators.
6
These drugs have various side effects, including muscle cramps, abnormal heart rate, blurred vision, skin rash, vomiting, kidney failure, extreme tiredness, headache, and edema.
7
Current growth in the acceptance of alternative medicines and natural products has drawn attention to traditional medicines for the treatment of CVDs.
8
Approximately 75% to 80% of the world’s population, predominantly in developing countries, uses herbal medicines for primary healthcare because of their better compatibility with the human body, lower costs than novel pharmaceuticals, and fewer side effects.
9
Persian medicine, an ancient and well-known traditional system of medicine, is based on the theory of humors for the prevention and treatment of diseases.
10
Persian medical scholars like Avicenna and Rhazes have described various types of diseases and recommended lifestyle modifications and herbal treatments for the alleviation of problems.
Medicinal plants have also been examined for their therapeutic properties. Some of them play an essential role in the production of over 50% of the currently available pharmaceutical drugs.
11
In this review article, a review of the diverse plants that have antihypertensive effects for use in the management of HTN is presented.
Pathophysiology of hypertension
The pathophysiological mechanisms implicated in the progress of HTN comprise raised vascular resistance, mainly distinguished through decreased vascular diameter because of enhanced vascular contraction and arterial remodeling.
12
Numerous factors contribute to the pathophysiology of HTN, including increases in the renin-angiotensin-aldosterone system (RAAS), stimulation of the sympathetic nervous system, vasopressin, disturbed G protein-coupled receptor signaling, inflammation, different T-cell roles, and the diversity of vasoactive peptides secreted by other endothelial cells and smooth muscle cells.
13
Increased arterial reactivity because of dysregulation in pro-oxidant enzymes and endothelial nitric oxide synthase (eNOS), increased basal and activated calcium levels via calcium channels, and co-occurrence of vascular smooth muscle cell (VSMC) hyperplasia and hypertrophy can cause enhanced vasoconstriction.
14
Augmented vascular stiffness is conducive to HTN, and its problems, such as atherosclerosis, indicating that therapy must be focused on vascular stiffness instead of only the modulation of peripheral vascular resistance.
15,16
Angiotensin II (Ang II) is able to stimulate cell cycle progress.
17
Genetic diseases of renal sodium secretion, genetically associated ailments of the Na/Ca2+ exchange in the smooth muscles of arteries, and hormonal-neurogenic vasoconstriction are other possible causes of HTN.
18
Herbal medicines used for the treatment of hypertension
Many antihypertensive agents usedin the treatment of HTN have some side effects. Therefore, scientific studies recommend diverse lifestyle alterations and the use of suitable medicinal plants in its treatment.
19
Secondary metabolites of some herbs and spices display antihypertensive properties. Most herbal medicines control and reduce HTN by exerting antioxidant, anti-inflammatory, and anti-apoptosis properties, stimulating the eNOS-NO signaling pathway, suppressing endothelial permeability, and activating angiogenesis.
20
The mechanisms of some medicinal plants or their extracts in the management of HTN are shown in Figure 1.
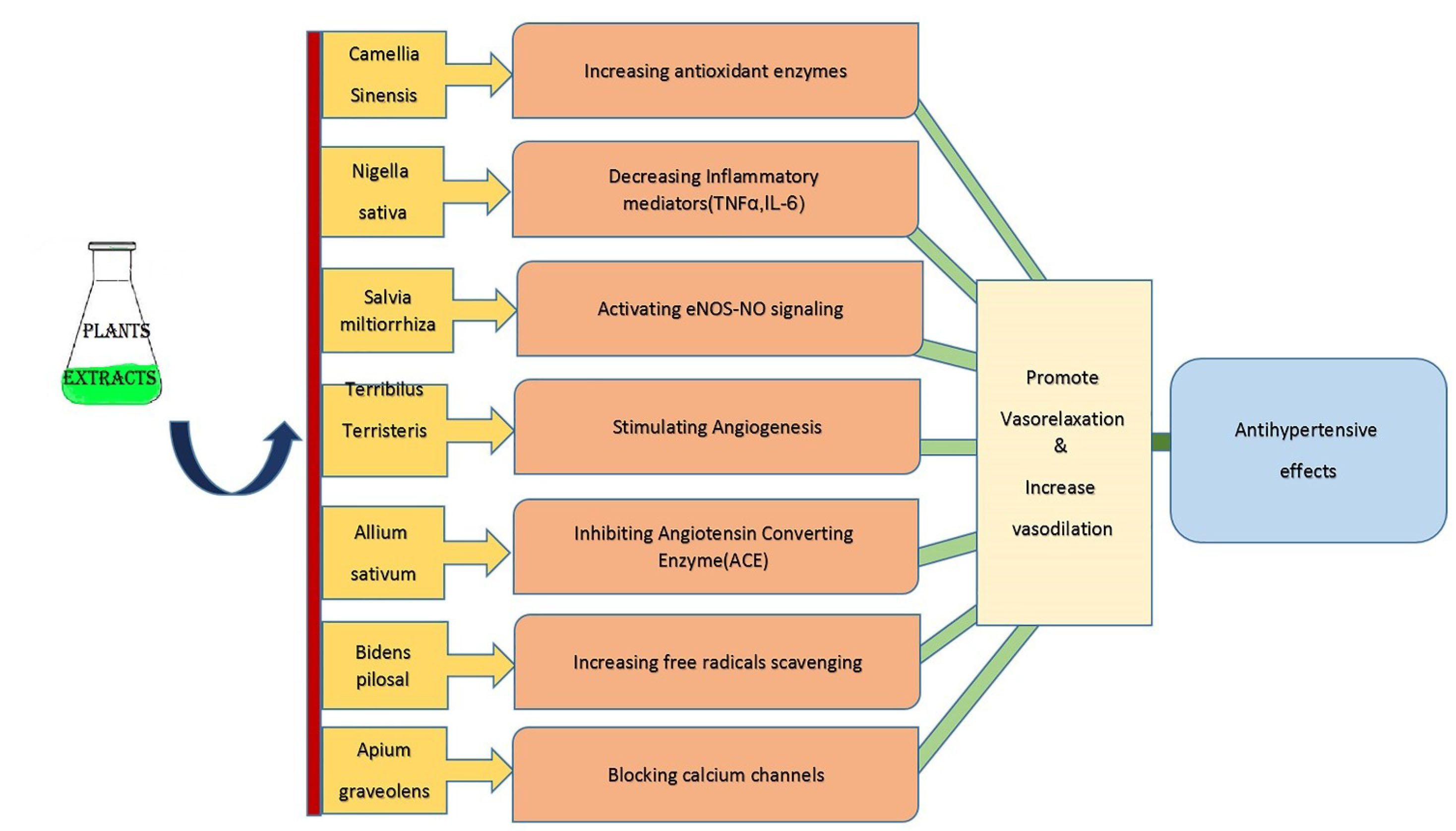
Figure 1.
Schematic diagram showing the mechanisms of some of medical plants or their extracts in the management of hypertension.
.
Schematic diagram showing the mechanisms of some of medical plants or their extracts in the management of hypertension.
Ajwain (Carum copticum L.)
Carum copticum belongs to the Apiaceae family and grows in various regions of Central Europe, Iran (particularly the eastern areas of Baluchistan), India, Afghanistan, and Pakistan.
21
As a result of its calcium channel blocking effect, C. copticum has a notable role in regulating heart rate and BP. The aqueous-methanolic extract of C. copticumBenth. seeds (CSE) (1-30 mg/kg) causes a decrease in BP and heart rate (HR) of normotensive (NMT) rats. At larger doses (10-30 mg/kg), bradycardia has been reported.
21
Bindii (Tribulus terrestris)
Tribulus terrestris is a medicinal plant used for treating HTN. Bindii causes a decrease in BP in spontaneously hypertensive (SHR) rats. Its methanolic and aqueous extracts (0.3–15 mg/mL) have been shown to have vasodilatory properties.
22
This plant is used for its diuretic effects. Furthermore, all of the saponins (furostanol and spirostanol saponins and sulphated saponins of tigogenin and diosgenin) of this plant prevent the production of H2O2 along with the proliferation of VSMCs.
23
Black Cumin (Nigella sativa)
The Nigella sativa plant, well recognized as the seed of blessing, has been used in the Middle East, Europe, and Africa for years. This plantand its components cause a decrease in BP.
24
Oral administration of N. sativa seed oil extract (100 or 200 mg) to mild hypertensive male patients for eight weeks results in a decline of 10.6 and 9.6 mm Hg in SBP and DBP, respectively.
25
Black cumin also lowers BP through vasorelaxation by means of its ability to block Ca2+ channels. Other mechanisms that may elucidate the hypotensive effect of N. sativa relate to its diuretic function, antioxidant activities, and anti-inflammatory properties.
26
Black-Jack (Bidens pilosa L.)
Black Jack, from the Asteraceae family, is an annual plant that grows in South America and is also found in tropical and subtropical regions around the world. Black Jack leaf extract was able to inhibit and reduce HTN in different rat models.
27
In fructose-fed rats, six hours after treatment with 75 and 150 mg/kg of methanolic leaf extract, SBP was decreased by 17% and 21%, respectively.
27
Additionally, B. pilosahas anti-cancer and anti-obesity effects as well as radical scavenging ability.
27
Black plum (Vitex doniana)
After oral administration of the fresh black plum fruit, both SBP and DBP were considerablydiminished in 45 minutes.BP began returning to standard after 2 hours.
28
Greater burdock (Arctium Lappa)
Burdock is also used for the treatment of HTN. This plant has reactive oxygen species (ROS) scavenging action, is able to inhibit vascular inflammation, and can stimulate vasorelaxation.
29
Arctigenin (a dietary phytoestrogen) is one bioactive component in the dry seeds of burdock that causes an increase in NO production and a decrease in the levels of superoxide anion.
30
Burhead (Echinodorus grandiflorus)
Echinodorusgrandiflorus is used in Brazilian folk medicine as a diuretic drug. The aqueous extracts of this plant can cause a decline in the mean arterial pressure (MAP) in addition to cardiac output and vascular resistance in SHRs. Burhead also induces persistent diuresis and decreased BP by activating muscarinic and bradykinin receptors with effects on prostaglandins and nitric oxide pathways.
31
Cardamom (Elettaria cardamomum)
Elettaria cardamomum fruit powder has been assessed for its antihypertensive capability. In powder form (3 g), it has been shown to reduce mean MAP as well as SBP and DBP by 19 and 12 mm Hg, respectively in pre-hypertensive subjects by increasing the total antioxidant status.
32
Carrot (Daucus carota L.)
Carrot has been used in traditional medicine as an antihypertensive mediator. Daucus carota L. improves endothelial function and regulates fluid balance. Carrot juice is rich in antioxidants, which decrease oxidative stress and control the function and structure of blood vessels. Carrots regulate BP because of the existence of potassium. Intravenous administration of the bioactive components of the aerial parts of D. carota, including DC-2 and DC-3, triggered a decrease in arterial BP in NMT rats. DC-2 and DC-3 can act by obstructing calcium channels.
12
Cat’s Claw herb (Uncaria rhynchophylla)
Cat’s claw is an herb used in traditional Chinese medicine to treat HTN. This plant causes a decrease in BP and relieves different neurological symptoms. Hirsutine (an indole alkaloid) is responsible for thehypotensive function of Uncariarhynchophylla, which decreasesintracellular Ca2+ levels through its effect on the Ca2+ store and its effects on the voltage-dependent Ca2+ channel.
33
Celery (Apium graveolens)
The seed extract of celery has been shown to have a BP-reducing effect in deoxycorticosterone acetate (DOCA)–induced hypertensive rats. The hexane extract is considerably more effective in reducing BP, probably by reducing levels of circulating catecholamines and diminishing vascular resistance. Extraordinarily, it has antioxidant effects due to the virtue of its flavonoid content.
34
Chakshushya (Cassia absus L.)
Cassia absus is a plant of the family Fabaceae with Ayurvedic ethnomedical records. This plant occurs in tropical areas and all over India. Intravenous administration of the alkaloid isolated from the seeds of Cassia absus Linn (1-30 mg/kg) reduces BP in rats. At higher doses (10 and 30 mg/kg), it causes a decline in HR. Frequent injection of a similar dose induces tachyphylaxis.
35
Chinese Sage (Salvia miltiorrhiza)
A traditional Chinese herb, Salvia miltiorrhiza, has been revealed to have cardioprotective effects on animals and humans. In addition to its vasodilatory capability, Chinese sage possesses anti-hypertensive properties including antioxidative effects through decreased ROS production, increased antioxidative enzymes, and anti-proliferative activities by preventing platelet-derived growth factor (PDGF)-induced proliferation of VSMCs, and anti-inflammatory capacity by inhibiting TNF-α and NF-κB production.
36,37
Cinnamon (Cinnamomum zeylanicum)
Another plant used for the treatment of HTN is Cinnamomum zeylanicum. Cinnamon has reduced BP in numerous rat models and in people with prediabetes and type2 diabetes (T2D). The aqueous extract of its stem bark causes a reduction in SBP and prevents contractions prompted by potassium chloride (also known as KCl), related to the endothelium, NO, and ATP-sensitive K+ channel (K ATP channel). The methanolic extract of the bark increases NO levels.
38
Cocoa Bean (Theobroma cacao)
Cocoa powder, augmented with flavonoidcomponents, is used for inhibiting CVDs by motivating the creation ofNO, increasing vasodilatation, and decreasing endothelialdysfunction. Daily use of dark or milk chocolate (40 to 105 g) can decreaseSBP by about 5 mm Hg and DBP by about 3 mm Hg.
39
Coffee Weed (Cassia occidentalis)
Coffee weed also decreases BP. The leaf of this plant is used as an antihypertensive agent. Coffee weed has been found to decrease BP levels, probably through the suppression of external Ca2+ influx. Coffee weed leaves have diuretic effects along with anti-inflammatory and anti-oxidant properties. They decrease lipid peroxide content and inhibit phospholipase A2 activity.
40
Coriander (Coriandrum sativum)
Coriander is used as a traditional medicine for the treatment of cardiovascular and gastrointestinal diseases. It has been shown to display antioxidant effects.
41
Intravenous use of the aqueous methanolic extract of the seeds (1–30 mg/mL) causes a reduction in SBP, DBP, and MABP, possibly through the Ca2+ antagonist. Additionally, this extract exhibits diuretic affects.
42
Dogbane (Apocynum venetum)
The leaves of the dogbane plant seem to be rich in flavonoids and quercetin variants, which have been found to help fight HTN. Extracts of dogbane leaves (10 μg/mL) induce vasorelaxation by enhancing NO, causing the scavenging of ROS. This plant’s extracts improve renal function as an antihypertensive effect.
43
Dog-strangling Vine (Cynanchum wilfordii)
Cynanchum wilfordii is used in traditional Chinese medicine, and nearly all parts of this plant are considered advantageous for different vascular diseases. Ethanolic extracts (100 and 200 mg/kg/d) of C. wilfordii reduced BP in high fat/cholesterol-fed rats, by motivating Akt, triggering increased eNOS activity as well as increased NO and cyclic guanosine monophosphate (cGMP) production in addition to a decline in the expression of VCAM-1 and endothelin-1 (ET-1).
44
Harmel (Peganum harmala)
Wild Syrian rue (family Zygophyllaceae) is called “Espand” in Persian, and different parts of this plant including its seeds, bark, and root have been used in folk medicine.
45
Espand is used for the treatment of HTN. Peganum harmala prompts relaxation through both endothelial cells and VSMCs. Three harmala alkaloids, i.e. Harmine, harmaline, and harmalol, are espand’s active constituents which have shown vasodilatory properties by increasing NO production.
46
Fang Ji (Stephania tetrandra)
Stephania tetrandra is able to regulate high BP by reducing inducible nitric oxide synthase (iNOS) expression and blocking Ca2+ channels. An alkaloid tetrandrine, the bioactive constituent of this plant, has anti-inflammatory and anti-oxidant effects, both of which are probably involved in the plant’s antihypertensive effects.
47
Garden Cress (Lepidium sativum L.)
The hypotensive effect of garden cressis associated with the augmented urinary removal of sodium, potassium, and chlorides. Lepidium sativum has been revealed to have anti-inflammatory effects. Lepidium sativum induces diuresis and effective antioxidant capability, to which its antihypertensive effects may be ascribed.
48
Garden Nasturtium (Tropaeolum majus L.)
Garden nasturtium belongs to the family Tropaeolaceae. Studies have confirmed that Tropaeolum majus has a positive influence on the circulatory system. Hydroethanolic extracts of garden nasturtiumhave been revealed to decrease MAP in SHR rats. The ethanolic extract of T. majus(300 mg/kg), cure element (100 mg/kg), or isoquercitrin (10 mg/kg), have diuretic activities. All the above-mentioned constituents are able to reduce plasma ACE levels. Isoquercitrin (an active flavonoid) causes the growth of NO production.
49
Garlic (Allium sativum)
Garlic supplements have revealed their effectiveness in the treatment of HTN, decreasing BP by about 10 mm Hg systolic and 8 mm Hg diastolic, like standard BP medication. This herb is recognized for its antibacterial, antioxidant, anti-inflammatory, anti-cancer, and hypocholesteremic effects.
50
One study displayed that garlic had an approximately 80% effectiveness in the treatment of HTN. Aged garlic extract (AGE) induces a constant drop in BP compared to with other forms of garlic. Furthermore, garlic supplements prompt a major decrease in both SBP and DBP by 3.75 and 3.39 mm Hg, respectively.
51
In another study, patients with HTN who ingested garlic tablets (300–1500 mg/d) for 24 weeks described a considerable reduction in SBP by 9.2 mm Hg and DBP by 6.27 mm Hg.
52
Moreover, AGE has superoxide scavenging abilities in human neutrophils, and daily use of 150 or 400 mg/kg of garlic extract prompted an increase in eNOS activity and a decline in nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase in the aortas of fructose-fed rats.
53
The components of garlic inhibit ACE activity, diminish Ang II-induced vasoconstrictor responses, prevent VSMCs proliferation in smooth muscles, antagonize endothelin-1 prompted vasoconstriction, and inhibit the stimulation of NF-κB.
54
Giant dodder (Cuscuta reflexa)
Cuscuta, commonly known as dodder, is a genus of the family convolvolaceace. The ethanolicextract of C. reflexa Led to a decline in SBP and DBP in anesthetized rats. In a dose-dependent manner, antihypertensive activity and bradycardia occurred.
55
Ginger (Zingiber officinale)
Zingiber Officinale, generally recognized as ginger, has been broadly used in the daily diet and for different therapeutic purposes. Ginger contains a large amount of potassium, which plays a role in the regulation of BP and heartbeat. Administration of two bioactive components of ginger, (6)-gingerol and (6)-shogaol, orally (70– 140 mg/kg) or intravenously (1.75–3.5 mg/kg) creates tri-phasic BP profiles: first a rapid drop, then an intermediate increase, and lastly, a delayed decline in BP. Currently, (6)-gingerol is considered to be a new Ang II type 1 receptor antagonist.
56
Recently, it has been found that ginger decreases levels of total cholesterol, triglycerides, low-density lipoprotein (LDL), and very low-density lipoproteins (VLDL). It also inhibits ACE-1 activity.
57
Ginseng (Panax spp.)
Ginseng is used in different forms, either as capsules, tablets, extracts, dried roots, oil, or as tea, and has hypotensive effects.
58
Small doses of ginsengincrease BP, whereas higher doses are hypotensive. Thus, ginsengregulates BP levels in hypotensive patients probably through vascular function change, controlling the autonomic nervous system, or adjusting the arterial baroreflex.
58
The panax ginsengextract in mild hypertensive patients induces a considerable decline of 3.1 mm Hg in SBP and mm Hg in 2.3 DBP.
59
Ginsenoside Rg3(red ginseng) stimulates eNOS, enhances NO and cGMP levels, and stimulates Ca2+ - gated K+ channels. Moreover, ginsenghas an anti-proliferative influence on VSMCs, and it has antihypertensive and anti-atherosclerotic abilities.
60
Red ginseng also reduces Ang II-induced VSMC growth. Another hypotensive mechanism of ginseng is its antioxidant ability, perhaps by increasing antioxidant enzymes and scavenging free radicals. Furthermore, ginsengdisplays anti-inflammatory properties by protecting the release of TNF-α and decreasing NF-κB and p38 MAPK pathways.
60
Goldthread (Coptis chinensis)
Goldthread and its most important constituent Berberine (BBR) can decrease BP. Coptis chinensis can block Ca2+ channels and inhibit cardiac hypertrophy. BBR can cause a significant decline in SBP (by an average of 4.91 mm Hg) and DBP (2 mm Hg).
61
BBR (150 mg/kg) can also scavenge ROS, prevent NADPH oxidase, and increase the antioxidant enzymes and superoxide dismutase (SOD).
62
BBR increases the expression of eNOS with a simultaneous increase in NO release that causes increased vasodilation. Furthermore, BBR prevents endothelial injury and controls inflammatory pathways by inhibiting NF-κB, VCAM-1expression and VSMC proliferation.
62
Gumbo limbo (Bursera simaruba)
Bursera simaruba, usually known as gumbo-limbo, is native to the tropical regions of the Americas. B. simaruba was shown to decrease heart rate and cause long-standing hypotension after one oral administration of the extract. B. simaruba also advanced the endothelial function by activating vascular endothelial NO synthase, thereby explaining the plant’s vascular protective influence.
63
Hardy fuchsia (Fuchsia magellanica)
Fuchsia magellanica is found in Chile and Southern Argentina. The leaf extract of this plant decreases body heat, has diuretic effects, and reduces BP. In NMT rats, ethanol/aqueous extracts of this species caused a significant decrease in MAP.
64
Hawthorn (Crataegus spp.)
Hawthorn plants have been used for the treatment of CVDs. Patients with mild HTN who were treated with 500 mg of hawthorn extract for ten weeks displayed a decrease by 13.1 mm Hg decrease in DBP.
65
Quercetin, a main compound in hawthorn shrubs, has antioxidant, anti-inflammatory, and vasorelaxant effects. Remarkably, hawthorn extracts affect both VSMCs and endothelial cells.
66
Furthermore, they have anti-inflammatory activity by decreasing the concentrations of NF-κB, TNF-α, VCAM-1, iNOS, and IL-6.
67
Indian Plantago (Plantago ovata)
Indian Plantago is an herb, the seed and outer covering of the seed (husk) of which are used to make medicine. A primary clinical study indicated that using 15 gof Plantago ovata supplement everyday could relatively reduce BP: SBP by around 8 mm Hg and DBP by 2 mm Hg.
68
Indian snakeroot (Rauwolfia serpentina)
Rauwolfia serpentina is a tropical woody plant used for the treatment of HTN by reducing the levels of dopamine and epinephrine and by promoting vasodilation. Reserpine, the main alkaloid of Rauwolfia serpentina, is the primary powerful drug broadly used in the longstanding treatment of HTN. In 1952, isolated reserpine was made known as the drug Serpasil for the treatment of HTN, tachycardia, and thyrotoxicosis.
69
Japanese Thistle (Cirsium japonicum)
Cirsium japonicum is a perennial herb native to Japan, China, and Korea. The aqueous extract (0.1–1.0 mg/mL) of this plant induces vasorelaxation by activating histamine H1 receptors. The principal mechanism includes elevated levels of NO and cGMP. Silibinin (0.05–0.4 mg/mL), a component of Japanese thistle, is an antagonist for the human angiotensin II receptor type 1 (AT1 receptor), so it can reduce SBP.
70
King of Bitters (Andrographis paniculata)
King of bitterhas been used in Asian traditional medicine for the treatment of CVDs.
71
The extracts of A. paniculata has been shown to lower ACE and ROS activities in SHR rats and cause a reduction in BP. The crude extract of A. paniculate, a compound of 14-deoxy-11,12-didehydroandrographolide, prompts considerable hypotensive properties by increasing NO release and inhibiting the rise in intracellular Ca2+.
72
It has been revealed to have anti-inflammatory, anti-bacterial, and antioxidant effects.
72
Kudzu (Pueraria lobata)
Pueraria lobata reduced BP in dogs and hypertensive patients through its vasodilatory effect, with its ability to stimulate Ca2+-activated K+ (KCa)channels.
73
This plant has anti-inflammatory and anti-oxidant activities, which can relatively explain its anti-hypertensive effects. Puerarin is the major bioactive compound in this plant which has antihypertensive and other cardioprotective properties.
74
Large-fruited elm (Ulmus macrocarpa)
Oral administration of 100 mg/kg root bark of Ulmus macrocarpa (RBUM) reduced SBP in SHR rats by 20 mm Hg. The anti-hypertensive influence of RBUM could be due to its ability to improve structural and functional modifications of vascular endothelium.
75
Lemongrass (Cymbopogon citratus)
Lemongrass is a plant whose leaves and oil are used to make medicine. Lemongrass is widely used in Southern Asia, China, and Brazil. Its antihypertensive effects have been ascribed to Citral, its active phytochemical compound.
76
Citralor crude extracts cause dose-dependent vasorelaxation through the activation of NO and the suppression of calcium channels. Lemongrass exerts modest antioxidant activity by suppressing ROS molecules and is involved in anti-inflammatory pathways by preventing NF-κB and iNOS activity.
77
Logolai (Bag.) (Viscum articulatum Burm.f.)
Viscum articulatum Brum.f. methanolic extract has diuretic properties. Furthermore, oleanolic acid extracted from Viscum articulatum Brum.f.(60 mg/kg/d) can raise NO levels in plasma. This plant also has antioxidant potential.
78
Makandi (Coleus forskohlii)
The Coleus forskohlii plant is a strong adenylyl cyclase activator. Makandi raised intracellular levels of cAMP, triggering the activation of protein kinase A(PKA), which consecutively prompted the relaxation of VSMCs, thereby causing a decrease in BP.
79
Maritime Pine (Pinus pinaster)
Pycnogenol (200 mg/d), an extract from French maritime pine bark, can relatively reduce BP inpeople with mild HTN, possibly by preventingangiotensin-converting enzymes.
80
Mistletoe (Agelanthus dodoneifolius)
Ethanolic extracts of mistletoe (0.01–10 mg/mL) reduced SBP and DBP in normotensive rats. The dodoneine mechanism induced vasorelaxation by preventing carbonic anhydrase and stimulating KCa channels.
81
Melon-Gubat (Melothria maderaspatana)
Melothria maderaspatana causes a reduction in BP in hypertensive humans. The use of melon-gubat tea for 45 days in subjects with mild HTN resulted in a substantial reduction by 23.8 mm Hg and 15.5 mm Hg in systolic and diastolic BP, respectively.
82
Murungai (Moringa oleifera)
The crude extract of the leaves of the Murungai plant triggers a decreasein SBP, DBP, and MBP in a dose-dependent manner by lessening vascular dysfunction and oxidative stress and stimulating endothelium-dependent vasorelaxation.The antihypertensive activity has been attributed to the thiocarbamate andisothiocyanate elements of the purified extract.
83
Onion (Allium cepa)
Onion was shown to decrease BP in fructose-fed and anesthetized normotensive rats.
84
Organo-sulfur compounds have been correlated with reducing BP by sustaining the elasticity of the major arteries accompanied by lowering the blood viscosity, thereby preventing blood clotting.
85
Quercetin, the composite most usually related to onions, can decrease BP an average of 5 mm Hg by decreasing oxidative stress through its reaction with free radicals and progressing vascular function.
85
Aqueous extracts of onion (400 mg/kg/d) increased eNOS expression but decreased that of VCAM-1. The antioxidant effects of onion seem to be the result of the inhibition of NADPH oxidase activity together with a simultaneous rise in antioxidant kinetics of glutathione peroxidase (GPX) enzymes and SOD.
86
Pointed Phoenix Tail (Gynura procumbens)
In Thai, Gynura procumbens is called “longevity spinach,” and in Chinese, it is called “Pointed Phoenix Tail.” The aqueous extract of pointed phoenix taildecreases BP in SHRs. In rat aortic rings, it inhibited contractions induced by Ang I and Ang II through a NO-dependent mechanism and inhibited ACE activity.
87
Furthermore, the crude extract of this plant (0.003 and 0.009 g/mL) suppressed both KCl- and phenylephrine-induced contractions, associated with the opening of K channels, preventing the Ca2+ channels and discharge of prostacyclin, so it displayed a vasodilatory effect.
88
Pomegranate (Punica granatum)
The pomegranate is a fruit-bearing deciduous shrub in the family Lythraceae that grows in the region extending from Iran to northern India. Pomegranatedecreases the activity of ACE by nearly 36%. One study displayed a modest decrease inSBP after drinking 50 ml/day of its juice for ayear.
89
Prickly Custard apple (Annona muricata).
Annona muricata is a species of the custard apple tree family. Annonaceae, which has edible fruit. A. muricata is native to Central America and the Caribbean. The methanolic extract of the A. muricata leaf has been described to decrease a raised BP by reducing peripheral vascular resistance.
90
Qingxue Dan (Chunghyul-dan)
Chunghyul-dan is an herbal complex that has anti-hypertensive effects on stroke patients with stage 1 HTN. In stroke patients, after the administration of 1200 mg Chunghvul-dan, SBP and DBP were considerably reduced in comparison with baseline.
91
Radish (Raphanus sativus)
Radish is an edible root vegetable of the family Brassicaceae, grown and used throughout the world. The leaf ethyl acetate extract (30 and 90 mg/kg) reduced SBP in SHRs, whereas the seeds (0.1–3 mg/kg) reduced BP along with HR. Radish extracts increase NO production and raise antioxidant levels. The anti-proliferative and anti-inflammatory capabilities of the radish may be partially involved in its antihypertensive effect.
92
Roselle (Hibiscus sabdariffa)
Hibiscus sabdariffa L. (HS) tea is used as a beverage and a treatment for HTN and hyperlipidemia. In patients with HTN, treatment with the dried extract of the calyx (250 mg) for 4 weeks has displayed remarkable antihypertensive effects.
93
After four weeks of ingesting 10 g/d of hibiscus calyx, the SBP and DBP of hypertensive patients was decreased significantly by 15.32 and 11.29 mm Hg, respectively. In mild and pre-hypertensive patients, using hibiscus tea (240 ml) 3 times daily for six weeks decreased SBP, DBP, and MAP considerably by 7.2, 3.1, and 4.5 mm Hg, respectively.
94
Its effects were facilitated through the elevated production of NO, blocking of Ca2+ channels, and opening of KATP channels. Roselle has diuretic effects, exhibits potent antioxidant function, and prevents the oxidation of LDL. Moreover, it shows anti-inflammatory capabilities through the prevention of ACE activity and proliferation of VSMCs.
95
Safflower (Carthamus tinctorius L.)
Carthamus tinctorius L., known as Kafesheh (Persian), is used extensively for numerous medical conditions including cerebrovascular and CVDs in traditional Chinese medicine. Safflower yellow (SY) reduced BP by opening KATP channels in addition to decreasing renin activity and Ang II levels in SHRs. In addition to reducing BP in healthy humans, the seed extract (2.1 g daily) also reduces both VCAM-1 and LDL levels, prevents PDGF-induced proliferation of VSMCs, and decreases arterial stiffness.
96
Saffron (Crocus sativus)
Crocus sativus L., generally known as saffron, is a fragrant plant belonging to the Iridaceae family. This plant is native to Spain, Morocco, Greece, Iran, India, and Pakistan.
97
Administration of 400 mg of saffron tablets to healthy humans for seven days led to a decrease of 11 and 5 mm Hg in SBP and MAP, respectively. In male Wistar rats, crocin treatment (200 mg/kg for 7 days) caused a major drop in oxidative stress and an increase in antioxidant enzymes.
98
Additionally, saffron and its components blocked the inflammatory pathways comprising NF-κB and TNF-α.
99
Sesame (Sesamum indicum)
Sesame is a flowering plant in the genus Sesamum. Sesame oil is a suitable prophylactic treatment for HTN. The alcoholic extract of the seeds (1–30 mg/kg) was shown to trigger a reduction in BP in anesthetized rats.
100
Shell Ginger (Alpinia zerumbet)
Shell Ginger, also known as bright ginger, is a perennial species of ginger from the family of Zingiberaceae. Alpinia zerumbet, a west Asian plant, has modest hypotensive properties. The vasorelaxant responses of methanolic fraction of the essential oil of shell ginger are induced through its effects on endothelial cells or VSMCs.
101
In DOCA-salt-treated rats, the methanolic extract of this plant’s leaves (100 and 300 μg/mL) prompted vasodilation by raising NO or cGMP production. 1–20 mg/kg of Alpinia zerumbet essential oil blocks Ca2+ channels, and 0.1 mg/L of this oil causes a decrease in the levels of oxidized LDL in plasma.
102
Stone breaker (Phyllanthus niruri)
Phyllanthus niruri is a plant that causes a reduction in BP in rabbits and humans. The aqueous extract of stone breaker(200 mg/kg) raises plasma antioxidants (GSH, GPx, SOD, and catalase CAT). Additionally, diverse solvent extracts of stone breakerhave been reported to prevent NF-κB, TNF-α, and COX-2.
103
Sumac (Rhus coriaria)
Sumac is a medicinal plant traditionally used for the treatment of CVDs. Rhus coriaria is known for its antioxidant activity. Hydrolysable tannins obtained from the leaves of sumac have been reported to display a vasorelaxant effect in an endothelium-dependent and NO-mediated manner. Importantly, this extract also has effective anti-inflammatory capabilities and can cause a decrease in TNF-α.
104
Sweet basil (Ocimum basilicum)
Ocimum basilicum L. is an herb used in traditional Chinese medicine to treat CVDs. In one study, the aqueous extract reduced BP levels in rats in a dose-dependent manner (100–400 mg/kg). It also induced a vasorelaxant effect and had ROS scavenging ability.
105
Sweet flag (Acorus calamus)
Sweet flag is a commonly known drug in the traditional system of medicine. The solvent extracts of sweet flagcaused a reduction in MAP in normotensive rats. Acorus calamus has vasoconstrictive or vasodilatory properties in rabbit aorta as well, possibly due to a Ca2+dependent mechanism.
106
Sweet violet (Viola odorata)
Viola odorata, commonly known as sweet violet, is native to Europe and Asia. The leaf extract of sweet violet (0.1, 0.3, and 1 mg/kg) lowered the MAP of rats. The extract induces relaxation and NO production, and Ca2+ influx control causes this vasodilatory effect. The extract also improves CVDs risk factors by stimulating a substantial decline in total cholesterol and LDL-C.
107
Tea (Camellia sinensis)
Tea is a beverage of cured leaves or leaf buds of the tea plant Camellia sinensis.
108
It has pleiotropic effects comprising antibacterial, anti-inflammatory, anti-cancer, and anti-diabetic properties, accompanied by antihypertensive actions. Green tea decreases both SBP and DBP by 1.98 and 1.92, respectively.
109
Remarkably, it has been stated that green tea induces a more potent hypotensive effect than black tea.
110
One study established that hypertensive patients who used up to 4479 mg of black tea for 24 weeks showed a substantial decrease by 2 and 2.1 mm Hg in SBP and DBP, respectively.
111
The bioactive constituents of tea have been shown to exert anti-oxidant and anti-inflammatory effects. The mechanisms of oxidative stress reduction by tea which include increasing CAT antioxidant enzyme, inhibition of eNOS uncoupling, superoxides scavenging capacity, and reducing NAPDH oxidase production, cause a reduction in BP besides TNF-α levels.
108
Epigallocatechin gallate (EGCG), derived from tea, caused a decrease in VCAM-1 levels, prevented NF- κB activation, and stimulated prevention of proliferation in human aortic VSMCs through the upregulation of HO-1 enzyme expression.
112
Tianma (Gastrodia elata Blume)
Gastrodia elata is a saprophytic perennial herb of the family Orchidaceae and is used in traditional Chinese medicine.Gastrodia rhizome has antihypertensive properties. The acidic polysaccharides extracted from the rhizome trigger a significant reduction in BP levels.
113
The methanolic extracts (0.02 ml/g) of Tianmaexhibited anti-inflammatory properties by decreasing iNOS expression and NO levels. In old patients with refractory HTN, gastrodin, a main bioactive constituent of Tianma, triggered a decline in SBP and pulse pressures, raised NO levels, and decreased endothelin levels. Gastrodin (a phenolic glycoside) decreased SBP by interfering with the RAAS and diminished serum levels of Ang II along with the expression of both ACE and AT1R.
114
Tomato (Lycopersicon esculentum)
The tomato is the edible part of the plant Solanum lycopersicum. Tomato extract contains carotenoids which are recognized as operative antioxidants. The extract of tomato (Lyc-O-Mato) moderately decreased BP in patients with HTN.
115
Tomato extract has a clinically substantial capacity to decrease SBP by more than 10 mm Hg and DBP by more than 5 mm Hg.
115
The root extract of tomato reduced BP levels in hypertensive rats. The antioxidant-rich extract of tomato has been revealed to decrease both SBP and DBP in hypertensive patients.
116
Turmeric (Curcuma longa)
Curcuma longa or turmeric, originates from Southeast India and is widely cultivated in the tropical areas of South Asia. Turmeric, also called curcumin, has anti-inflammatory and anti-cancer properties.
117
Curcumin exerts advantageous effects on CVDs, such as HTN. Curcumin decreases AT1R expression in arteries by disturbing SP1/AT1R DNA binding, thereby decreasing AT1R-mediated vasoconstriction and then inhibiting the progress of HTN.
118
Umbrella tree (Musanga cecropioides)
Musanga cecropioides, the African corkwood or umbrella tree, is found throughout the tropical rain forests, mostly in West Africa. The latex and the leaf extract of this plant are used as a vasorelaxant and a hypotensive mediator. The water extract of the stem bark produces a dose-dependent decrease in MABP at the dose of 10 mg/kg (4.51 ± 0.5 mm Hg) and at the 40 mg/kg dose (65.23 ± 6.28 mm Hg).
119
Vidanga (Embelia ribes)
Embelia ribes, commonly known as false black pepper, is a species in the family Primulaceae. It is widely dispersed throughout India.Embeliaribes has hypotensive effects. The aqueous extract of E. ribes (100 mg/kg) is able to reduce both SBP and HR and enhance endogenous antioxidants, including SOD, CAT, and GSH.
120
White Horehound (Marrubium vulgare)
Marrubium vulgare (common horehound) is a flowering plant native to Europe, northern Africa, and southwestern and central Asia. White horehound causes a significant decrease in SBP. This hypotensive effect may be due to its anti-hypertrophic and vasorelaxant properties. The diterpene marrubenol isolated from this plant can strongly block L-type Ca2+ channels and subsequently prevent the contraction of VSMCs. Also, phenylpropanoids extracted from white horehound can prevent the lipoprotein-induced secretion of endothelin-1.
121
Some medicinal plants which are commonly acknowledged to be beneficial in the treatment of HTN are discussed in Table 1.
Table 1.
Effective medicinal plants on hypertension
|
Herb
|
Mechanism of Action
|
Part used
|
Dose
|
References
|
|
Ajwain (Carum copticum)
|
-Blocks calcium channel
- Cholinomimetic effects
- Causes to vasodilation of coronary arteries
-Decreases systemic blood pressure
|
-Leaves
-Seed-like fruit
|
1-30 mg/kg |
21
|
|
Bindii (Tribulus terrestris)
|
- Increases NO
- Reduces ACE
- Inhibits Ang II-induced proliferation
|
-Leaves
-Aqueous extract of tribulus fruits
|
0.3–15 mg/mL |
23
|
|
Black Cumin (Nigella sativa)
|
-Reduces in cardiac oxidative stress
-Reduces angiotensin-converting enzyme activity
-Increases in cardiac heme oxygenase-1 activity
-Prevents of plasma nitric oxide loss
|
-Seeds oil |
100 mg/kg and
200 mg/kg
|
25
|
|
Black-Jack(Bidens pilosaL)
|
-Is calcium channel antagonism |
-Leaves |
75 and 150 mg/kg |
27
|
|
Burdock(Arctium Lappa)
|
- Suppresses VCAM-1 (aortic endothelia)
- Promotes vasorelaxation
|
- Root |
100 and 200 mg/kg/d |
29
|
|
Cardamom(Elettaria cardamomum)
|
- Blocks Ca2+ channels
- Increases urine output
-Enhances Na+ and K+ excretion
|
- Crude |
3 g/d |
32
|
|
Celery(Apium graveolens)
|
-Decreases levels of circulating catecholamines
-Reduces vascular resistance
-Blocks calcium channel
|
-Seeds |
300mg/kg |
34
|
|
Chinese Sage(Salviae miltiorrhizae)
|
- Increases NO
- Opens KATP channels
- Blocks Ca2+ channels
- Reduces ACE activity
|
- Dried root |
0–10 mg/mL |
37
|
|
CocoaBean(Theobroma cacao)
|
-Up-regulates NO
-Promotes vasodilation
-Improves endothelial function
|
- Cocoa Bean |
40 - 105 g |
39
|
|
Garden Nasturtium(Tropaeolum majus L)
|
- Downregulates ACE
- Increases NO
- Decreases aldosterone
- Reduces renal Na+/K+ pump
- Enhances urine volume
|
-Seeds
-Leaves
-Flowers
|
10-300 mg/kg |
49
|
|
Garlic(Allium sativum)
|
-Relaxes of blood vessels
-Reduces in the ability of blood to clot
- Increases NO
- Inhibits ACE
- Prevents Ang-II-induced cell cycle progression
|
-Fruits |
300–1500 mg/d |
52
|
|
Ginger(Zingiber officinale)
|
-Blocks Ca2+ channels
- Promotes vasodilation
|
- Root |
70– 140 mg/kg |
56
|
|
Ginseng (genus Panax)
|
-Enhances NO and cGMP levels
-Has an anti-proliferative influence on VSMCs
|
- Root |
3 g/d |
60
|
|
Japanese Thistle(Cirsium japonicum)
|
-Induces vasorelaxation
-Elevates levels of NO
-Is an antagonist for the AT1 receptor
|
-Whole plant |
0.05–0.4 mg/mL |
70
|
|
Onion (Allium cepa)
|
-Elasticity of arteries
-Decreases in blood viscosity
-Interfaces with Renin-Angiotensin System
-Improves of endothelial and vascular function
|
-Fruits |
400 mg/kg/d |
85
|
|
Pomegranate (Punica granatum)
|
-Enhances endothelium-dependent coronary relaxation
-Inhibits of calcium influx
-Reduces ACE activity
|
-Fruits |
50 mL/d |
89
|
|
Radish(Raphanus sativus)
|
- Increases NO production |
-Seeds
-Leaves -Root
|
30 and 90 mg/kg |
92
|
|
Roselle(Hibiscus sabdariffa)
|
- Enhances production of NO
-Inhibits of Ca2+ channels
-Opens of KATP channels
|
-Leaves
-Flowers
|
250 mg-10 g/d |
94
|
|
Saffron(Crocus sativus)
|
- Activates eNOS
- Blocks Ca2+ channels
|
-Stigma |
400 mg |
98
|
|
Sumac(Rhus coriaria)
|
- Evokes endothelium-dependent vasorelaxation
- Activates eNOS
|
- Leaves
- Fruits (red berries)
|
0.3–300 μg/mL |
104
|
|
Tea(Camellia sinensis)
|
-Inhibition of angiotensin converting enzyme
- Blocks Ca2+ channels
- Diuretic
-Enhances eNOS activity
|
- Leaves |
3 cups/day |
108
|
|
Turmeric (Curcuma longa)
|
-Interference with Ca2+ concentration
- Reduces ACE activity
- Reduces AT1 receptor expression
-Increase vasodilation
-Increase No production
|
- Root |
50-100 mg/kg/d |
118
|
Medicinal plants used for the treatment of HTN in Iran
The Sassanid Empire in Iran had an efficient and advanced official system of medicine that prominently influenced the advancement of medical sciences.
122
For the duration of the golden age of the Islamic era, from the 9th to the 12th centuries A.D., medical information from numerous fields concerning cardiology thrived because of outstanding Persian physicians and scholars.
123
Avicenna assumed and demonstrated that some natural medicaments have the capacity to help other treatments by directing them towards specific body organs.
124,125
In view of that, Avicenna suggested the combination therapy of a cardiac medicine with Lemon balm (Melissa officinalis L.) or Behmen (Centaurea behen L.).
122
The comparison of herbal medicines used in studying HTN in different regions of Iran has indicated that different parts of Iran use diverse plants to treat this disorder.
126
In Mobarakeh of Isfahan,curly dock (Rumex crispus L.),jujube (Ziziphus jujuba L.), and olive (Olea europaea L.) are used conventionally.
127
In Sistan and Baluchestan province, nigella (Nigella sativa L.) is used to treat HTN.
128
Milk thistle (Silybum marianum L.),yarrow (Achillea tenuifolia), chicory (Cichorium intybus), barberry (Berberis vulgaris), shepherd’s purse (Capsella bursa-pastoris), field horsetail (Equisetum arvense), Persian walnut (Juglans regia),and annual yellow sweetclover (Melilotus indicus)are used in Kazerun to treat HTN.
129
In the Arasbaran region, barberry,yarrow (Achillea millefolium L.), ecballium (Ecballium elaterium), common hawthorn (Crataegus monogyna), and English yew (Taxus baccata L.) are considered to have BP lowering potential.
130
Falcaria vulgaris (Falcaria vulgaris),saffron (Crocus haussknechtii), berberidaceae (Berberis integerrima), Christ’s thorn jujube, ramsons (Allium ursinum), salsify (Tragopogon porrifolius), and dill (Anethum graveolens) are used to treat high BP in Lorestan Province.
131
Warty-leaved rhubarb (Rheum ribes L.)and Christ’s thorn (Paliurus spina-christi) are used to decrease BP in Ilam province.
132
The bioactive substances of dill could be a source for anti-HTN and anti-diabetes properties.
133
Barberry has a lowering effect on BP. Valerian has BP diminishing effects in animals.
134
Medicinal plants that have been recognized as being effective in controlling and treating HTN are listed in Table 2.
128,134,135
Table 2.
Complete information of therapeutic effects of medicinal herbs in high BP in Iran
|
Scientific name
|
Persian Name
|
Usable Part
|
Region/Province
|
Preparation methods
|
|
Achillea millefolium L.
|
Boumadaran |
Shoot |
East Azerbaijan (Arasbaran) |
Decoction |
|
Allium sativum L.
|
Sir |
Root/ Bulb |
West Azerbaijan |
Fresh |
|
Allium ursinum
|
Valak |
Shoot |
Lorestan |
Raw or with food |
|
Althea aucheri Boiss.
|
Khatmi-Armanestani |
Aerial parts |
Fars |
Decoction |
|
Anthemis cotula L.
|
Babouneye bahari |
Flower |
North of Iran |
Decoction |
|
Anethum graveolens dhi
|
Shevid |
Whole parts |
Lorestan |
Dried or fresh with food |
|
Amygdalus scoparia
|
Badam |
Fruit |
Lorestan |
Sodden peel |
|
Berberis vulgaris L. /Berberis integrima
|
Zereshk |
Leaves and fruit |
East Azerbaijan (Arasbaran)
/ Lorestan
|
Cooked or sodden |
|
Camellia sinensis
|
Chay-sabz |
Leave |
North of Iran |
Decoction |
|
Capparis spinosa
|
Hendevaneh
aboujahl
|
Leaves and fruit |
Lorestan |
Raw fruit or dry leaf distillate is eaten |
|
Centaurea depressa M.
|
Golegandom |
Seed |
Fars |
Decoction |
|
Cichorium intybus L.
|
Kasni |
Leave |
East Azerbaijan |
Decoction |
|
Coriandrum sativum
|
Geshniz |
Leave |
East Azerbaijan |
Fresh |
|
Cotoneaster persica Pojark.
|
Shirkhest |
Aerial parts |
Fars (Kuh-Delu) |
Decoction |
|
Crataegus monogyna / Crataegus pontica C. Koch.
|
Zalzalak |
Leaves and fruit |
East Azerbaijan (Arasbaran)
/ Ilam
|
Fresh or cooked or sodden |
|
Descurainia sophia (L.) Schr.
|
Khakshir |
Fruit |
East Azerbaijan |
Fresh |
|
Echium amoenum L.
|
Gav zaban |
Flower |
Isfahan (Mobarakeh) |
Decoction |
|
Falcaria vulgaris
|
Ghaziaghi |
Flower
leaf, stem
|
Lorestan |
Leaves are cooked and eaten with food |
|
Ficus religiosa
|
Anjir |
Fruit |
Fars/ Lorestan |
Fresh |
|
Glaucium oxylobum
|
Shaghayegh-Goltiz |
Leave |
Golestan/Khorasan |
Decoction |
|
Glaucium grandiflorum
|
Shaghayegh-Goldorosht |
Leave |
Golestan/Khorasan |
Decoction |
|
Gundelia tournefortii L.
|
Kangar |
Leave |
Kurdestan |
Fresh |
|
Hypericum perforatum
|
Chay-Koohi |
Leave |
Kurdestan/ Azerbaijan / Ilam |
Decoction |
|
Juglans regia L.
|
Gerdou |
Leaves and fruit |
West Azerbaijan |
Fresh |
|
Morus alba
|
Toot |
Fruit |
Lorestan |
Raw berry or dried berry |
|
Matricaria recutita
|
Babooneh |
Flower |
Fars |
Decoction |
|
Nasturtium officinale R.
|
Alafe cheshme |
Shoot |
Kurdestan(Marivan) |
Decoction |
|
Nectaroscordeum tripedale/ Nectaroscordeum coelzi
|
Piaze tabestaneh lorestani |
Shoot |
Lorestan |
Fresh |
|
Nigella sativa L.
|
Siah daneh |
Seed |
Sistan |
Fresh |
|
Olea europaea L.
|
Zeytoon |
Leave and Fruit |
North Iran |
Decoction |
|
Paliurus spina-christi Miller.
|
Siah tale |
Fruit |
Ilam |
Fresh |
|
Petroselinum sativum
|
Jafari |
Leave |
East Azerbaijan |
Fresh |
|
Physalis alkekengi
|
Aroosak-Poshtpardeh |
Aerial parts |
Khuzestan |
Decoction |
|
Rheum ribes L.
|
Rivas |
Stem |
Ilam |
Fresh |
|
Rhus Coriaria. L.
|
Somagh |
Fruit |
Kurdestan |
Decoction |
|
Ribes divaricatum /Ribes orientale
|
Angoor |
Leave and Fruit |
East Azerbaijan
(Maragheh/Arasbaran)
|
Fresh |
|
Rumex pulcher L. / Rumex crispus L./ Rumex conglomerates Murr
|
Torshak |
Root/ Leaves and stem |
Khuzestan/ Isfahan |
Fresh |
|
Securigera securidaca Degen & Dorfl.
|
Adas talkh |
Seed |
Khuzestan |
Fresh |
|
Silybum marianum L. / Silybum marianum (L.)Gaerth.
|
Khar maryam |
Stem and root/ Flower |
Khuzestan/Fars |
Decoction |
|
Smyrnium cordifolium
|
Andol |
Seed |
Lorestan |
Squeezed seeds |
|
Suaeda altissima
|
A type of Siah shor |
Leaves and stem |
North East Persian Gulf |
Decoction |
|
Tragopogon aureus Boiss
|
A type of Sheng |
Leaves and fruit |
Khuzestan |
Fresh |
|
Trigonella monspeliaca
|
Shanbalileh-Monileei |
Leave and fruit |
Fars |
Fresh |
|
Valeriana officinalis
|
Sonboletib |
Aerial parts |
Isfahan |
Decoction |
|
Viscum album
|
Darvash |
Aerial parts |
Khuzestan |
Decoction |
|
Ziziphus jujuba (L)H.Karst
|
Anab |
Fruit |
Isfahan |
Fresh |
|
Ziziphus nummularia
|
Konar |
Bulb |
Lorestan |
Fresh |
|
Ziziphus spina-christi
|
Sedr |
Leaves, flowers and fruit |
Lorestan |
Sodden leaves and flower |
Study limitations
A small number of traditionally used plants have been confirmed precisely through animal studies and clinical trials, but the detailed mechanisms of action of these plants are still unknown. Medicinal plants are unsuccessful in attaining the anticipated scale due to a shortage of scientific data on their safety and efficiency. Thus, systematic validation studies are required.
Conclusion
Avicenna had a remarkable influence on the field of cardiology, and his role had the most prominent effects on the progress of cardiological science. In the third volume of the Canon of Medicine, Avicenna defined numerous cardiovascular conditions and disorders. HTN is among the most prevalent diseases in the world, though it can be regulated and prohibited, and causes many difficulties for affected patients. Many simple approaches can be adopted to regulate high BP, such as lifestyle changes, pharmacotherapy, or both.
Future view
Traditional botanical research on medicinal plants suggests novel areas of study on the antihypertensive effects of medicinal plants. With regard to their safety and efficacy, medicinal plants can be processed to produce natural medications; however, their effect should be confirmed by pharmacological research and clinical trials. Studies in the future, which will focus on elongated randomized trials, may be of assistance in clarifying the durable effects of medicinal plants. In addition, studies on different herbs with antihypertensive effects have been promising so far and will lead to the discovery of new antihypertensive herbal medicines in the near future.
Ethical Issues
Not applicable.
Conflict of Interest
Authors declare no conflict of interest.
Acknowledgments
Authors would like to acknowledge Department of Persian Medicine, Faculty of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz, Iran for their great help.
References
- Al Disi SS, Anwar MA, Eid AH. Anti-hypertensive herbs and their mechanisms of action: part I. Front Pharmacol 2015; 6:323. doi: 10.3389/fphar.2015.00323 [Crossref] [ Google Scholar]
- Wang J, Xiong X. Control strategy on hypertension in Chinese medicine. Evid Based Complement Alternat Med 2012; 2012:284847. doi: 10.1155/2012/284847 [Crossref] [ Google Scholar]
- Anwar MA, Al Disi SS, Eid AH. Anti-hypertensive herbs and their mechanisms of action: part II. Front Pharmacol 2016; 7:50. doi: 10.3389/fphar.2016.00050 [Crossref] [ Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011; 123(4):e18-e209. doi: 10.1161/CIR.0b013e3182009701 [Crossref] [ Google Scholar]
- Hashemi V, Dolati S, Hosseini A, Gharibi T, Danaii S, Yousefi M. Natural killer T cells in preeclampsia: an updated review. Biomed Pharmacother 2017; 95:412-8. doi: 10.1016/j.biopha.2017.08.077 [Crossref] [ Google Scholar]
- Sinha AD, Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in CKD. Clin J Am Soc Nephrol 2019; 14(5):757-64. doi: 10.2215/cjn.04330418 [Crossref] [ Google Scholar]
- Singh P, Mishra A, Singh P, Goswami S, Singh A, Tiwari KD. Hypertension and herbal plant for its treatment: a review. Indian J Res Pharm Biotechnol 2015; 3(5):358-66. [ Google Scholar]
- Rastogi S, Pandey MM, Rawat AK. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine 2016; 23(11):1082-9. doi: 10.1016/j.phymed.2015.10.012 [Crossref] [ Google Scholar]
- Agrawal M, Nandini D, Sharma V, Chauhan NS. Herbal remedies for treatment of hypertension. Int J Pharm Sci Res 2010; 1(5):1-21. doi: 10.13040/ijpsr.0975-8232.1(5).1-21 [Crossref] [ Google Scholar]
- Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech 2019; 9(1):4. doi: 10.1007/s13205-018-1528-0 [Crossref] [ Google Scholar]
- Shayganni E, Bahmani M, Asgary S, Rafieian-Kopaei M. Inflammaging and cardiovascular disease: management by medicinal plants. Phytomedicine 2016; 23(11):1119-26. doi: 10.1016/j.phymed.2015.11.004 [Crossref] [ Google Scholar]
- Kaur R, Khanna N. Pathophysiology and risk factors related to hypertension and its cure using herbal drugs. Spatula DD 2012; 2(4):245-56. doi: 10.5455/spatula.20121223101221 [Crossref] [ Google Scholar]
- Rawat P, Singh PK, Kumar V. Anti-hypertensive medicinal plants and their mode of action. J Herb Med 2016; 6(3):107-18. doi: 10.1016/j.hermed.2016.06.001 [Crossref] [ Google Scholar]
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 2012; 95(2):194-204. doi: 10.1093/cvr/cvs135 [Crossref] [ Google Scholar]
- Sehgel NL, Zhu Y, Sun Z, Trzeciakowski JP, Hong Z, Hunter WC. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 2013; 305(9):H1281-7. doi: 10.1152/ajpheart.00232.2013 [Crossref] [ Google Scholar]
- Rostamzadeh D, Razavi SR, Esmaeili S, Dolati S, Ahmahi M, Sadreddini S. Application of nanoparticle technology in the treatment of systemic lupus erythematous. Biomed Pharmacother 2016; 83:1154-63. doi: 10.1016/j.biopha.2016.08.020 [Crossref] [ Google Scholar]
- Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med 2012; 52(9):1607-19. doi: 10.1016/j.freeradbiomed.2012.01.025 [Crossref] [ Google Scholar]
- Zhang Y, Jose PA, Zeng C. Regulation of sodium transport in the proximal tubule by endothelin. Contrib Nephrol 2011; 172:63-75. doi: 10.1159/000328684 [Crossref] [ Google Scholar]
- Pourjabali M, Mohammadrezaei-Khorramabadi R, Abbaszadeh S, Naghdi N, Naji-Haddadi S, Bahmani F. Medicinal plants used for hypertension. J Pharm Sci Res 2017; 9(5):537-41. [ Google Scholar]
- Liu C, Huang Y. Chinese herbal medicine on cardiovascular diseases and the mechanisms of action. Front Pharmacol 2016; 7:469. doi: 10.3389/fphar.2016.00469 [Crossref] [ Google Scholar]
- Boskabady MH, Alitaneh S, Alavinezhad A. Carum copticum L: a herbal medicine with various pharmacological effects. Biomed Res Int 2014; 2014:569087. doi: 10.1155/2014/569087 [Crossref] [ Google Scholar]
- Kumar K, Sharma YP, Manhas RK, Bhatia H. Ethnomedicinal plants of Shankaracharya Hill, Srinagar, J&K, India. J Ethnopharmacol 2015; 170:255-74. doi: 10.1016/j.jep.2015.05.021 [Crossref] [ Google Scholar]
- Sharifi AM, Darabi R, Akbarloo N. Study of antihypertensive mechanism of Tribulus terrestris in 2K1C hypertensive rats: role of tissue ACE activity. Life Sci 2003; 73(23):2963-71. doi: 10.1016/j.lfs.2003.04.002 [Crossref] [ Google Scholar]
- Leong XF, Rais Mustafa M, Jaarin K. Nigella sativa and its protective role in oxidative stress and hypertension. Evid Based Complement Alternat Med 2013; 2013:120732. doi: 10.1155/2013/120732 [Crossref] [ Google Scholar]
- Jaarin K, Foong WD, Yeoh MH, Kamarul ZY, Qodriyah HM, Azman A. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics (Sao Paulo) 2015; 70(11):751-7. doi: 10.6061/clinics/2015(11)07 [Crossref] [ Google Scholar]
- Kundu JK, Liu L, Shin JW, Surh YJ. Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem Biophys Res Commun 2013; 438(4):721-7. doi: 10.1016/j.bbrc.2013.07.110 [Crossref] [ Google Scholar]
- Bartolome AP, Villaseñor IM, Yang WC. Bidens pilosa L (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid Based Complement Alternat Med 2013; 2013:340215. doi: 10.1155/2013/340215 [Crossref] [ Google Scholar]
- Ladeji O, Udoh FV, Okoye ZS. Activity of aqueous extract of the bark of Vitex doniana on uterine muscle response to drugs. Phytother Res 2005; 19(9):804-6. doi: 10.1002/ptr.1588 [Crossref] [ Google Scholar]
- Lee YJ, Choi DH, Cho GH, Kim JS, Kang DG, Lee HS. Arctium lappa ameliorates endothelial dysfunction in rats fed with high fat/cholesterol diets. BMC Complement Altern Med 2012; 12:116. doi: 10.1186/1472-6882-12-116 [Crossref] [ Google Scholar]
- Cheng Y, Zhou M, Wang Y. Arctigenin antagonizes mineralocorticoid receptor to inhibit the transcription of Na/K-ATPase. J Recept Signal Transduct Res 2016; 36(2):181-8. doi: 10.3109/10799893.2015.1075039 [Crossref] [ Google Scholar]
- Prando TB, Barboza LN, Araújo Vde O, Gasparotto FM, de Souza LM, Lourenço EL. Involvement of bradykinin B2 and muscarinic receptors in the prolonged diuretic and antihypertensive properties of Echinodorus grandiflorus (Cham & Schltdl) Micheli. Phytomedicine 2016; 23(11):1249-58. doi: 10.1016/j.phymed.2015.10.020 [Crossref] [ Google Scholar]
- Verma SK, Jain V, Katewa SS. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J Biochem Biophys 2009; 46(6):503-6. [ Google Scholar]
-
Lamba A, Oakes AK, Roberts L, Deprele S. The Effects of Crude and Purified Cat’s Claw Extracts on Viability and Toxicity of HeLa Cells. Southern California Conference for Undergraduate Research (SCCUR); 2018.
- Siska S, Mun Im A, Bahtiar A, Suyatna FD. Effect of Apium graveolens extract administration on the pharmacokinetics of captopril in the plasma of rats. Sci Pharm 2018; 86(1):6. doi: 10.3390/scipharm86010006 [Crossref] [ Google Scholar]
- Ahmad S, Hassan A, Abbasi WM, Rehman T. Phytochemistry and pharmacological potential of Cassia absus - a review. J Pharm Pharmacol 2018; 70(1):27-41. doi: 10.1111/jphp.12816 [Crossref] [ Google Scholar]
- Cho YH, Ku CR, Hong ZY, Heo JH, Kim EH, Choi DH. Therapeutic effects of water soluble danshen extracts on atherosclerosis. Evid Based Complement Alternat Med 2013; 2013:623639. doi: 10.1155/2013/623639 [Crossref] [ Google Scholar]
- Jiang B, Li D, Deng Y, Teng F, Chen J, Xue S. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS One 2013; 8(3):e59621. doi: 10.1371/journal.pone.0059621 [Crossref] [ Google Scholar]
- Nyadjeu P, Nguelefack-Mbuyo EP, Atsamo AD, Nguelefack TB, Dongmo AB, Kamanyi A. Acute and chronic antihypertensive effects of Cinnamomum zeylanicum stem bark methanol extract in L-NAME-induced hypertensive rats. BMC Complement Altern Med 2013; 13:27. doi: 10.1186/1472-6882-13-27 [Crossref] [ Google Scholar]
- Irondi AE, Olawuyi AD, Lawal BS, Boligon AA, Olasupo F, Olalekan SI. Comparative inhibitory effects of cocoa bean and cocoa pod husk extracts on enzymes associated with hyperuricemia and hypertension in vitro. Int Food Res J 2019; 26(2):557-64. [ Google Scholar]
-
Ali M, Ansari SH, Ahmad S, Sanobar S, Hussain A, Khan SA, et al. Phytochemical and pharmacological approaches of traditional alternate Cassia occidentalis L. In: Ozturk M, Hakeem KR, eds. Plant and Human Health, Volume 3: Pharmacology and Therapeutic Uses. Cham: Springer; 2019. p. 321-41. 10.1007/978-3-030-04408-4_15
- Ramkissoon JS, Mahomoodally MF, Ahmed N, Subratty AH. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med 2013; 6(7):561-9. doi: 10.1016/s1995-7645(13)60097-8 [Crossref] [ Google Scholar]
- Wu TT, Tsai CW, Yao HT, Lii CK, Chen HW, Wu YL. Suppressive effects of extracts from the aerial part of Coriandrum sativum L on LPS-induced inflammatory responses in murine RAW 2647 macrophages. J Sci Food Agric 2010; 90(11):1846-54. doi: 10.1002/jsfa.4023 [Crossref] [ Google Scholar]
- Chen M, Zhao XY, Zuo X. Comparative reproductive biology of Apocynum venetum L in wild and managed populations in the arid region of NW China. Plant Syst Evol 2015; 301(6):1735-45. doi: 10.1007/s00606-014-1192-8 [Crossref] [ Google Scholar]
- Choi DH, Lee YJ, Kim JS, Kang DG, Lee HS. Cynanchum wilfordii ameliorates hypertension and endothelial dysfunction in rats fed with high fat/cholesterol diets. Immunopharmacol Immunotoxicol 2012; 34(1):4-11. doi: 10.3109/08923973.2011.569889 [Crossref] [ Google Scholar]
- Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev 2013; 7(14):199-212. doi: 10.4103/0973-7847.120524 [Crossref] [ Google Scholar]
- Cheraghi Niroumand M, Farzaei MH, Amin G. Medicinal properties of Peganum harmala L in traditional Iranian medicine and modern phytotherapy: a review. J Tradit Chin Med 2015; 35(1):104-9. doi: 10.1016/s0254-6272(15)30016-9 [Crossref] [ Google Scholar]
- Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J. Tetrandrine suppresses lipopolysaccharide-induced microglial activation by inhibiting NF-κB and ERK signaling pathways in BV2 cells. PLoS One 2014; 9(8):e102522. doi: 10.1371/journal.pone.0102522 [Crossref] [ Google Scholar]
- Fan QL, Zhu YD, Huang WH, Qi Y, Guo BL. Two new acylated flavonol glycosides from the seeds of Lepidium sativum. Molecules 2014; 19(8):11341-9. doi: 10.3390/molecules190811341 [Crossref] [ Google Scholar]
- Junior AG, Prando TB, Leme Tdos S, Gasparotto FM, Lourenço EL, Rattmann YD. Mechanisms underlying the diuretic effects of Tropaeolum majus L extracts and its main component isoquercitrin. J Ethnopharmacol 2012; 141(1):501-9. doi: 10.1016/j.jep.2012.03.018 [Crossref] [ Google Scholar]
- Shouk R, Abdou A, Shetty K, Sarkar D, Eid AH. Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res 2014; 34(2):106-15. doi: 10.1016/j.nutres.2013.12.005 [Crossref] [ Google Scholar]
- Wang HP, Yang J, Qin LQ, Yang XJ. Effect of garlic on blood pressure: a meta-analysis. J Clin Hypertens (Greenwich) 2015; 17(3):223-31. doi: 10.1111/jch.12473 [Crossref] [ Google Scholar]
- Ashraf R, Khan RA, Ashraf I, Qureshi AA. Effects of Allium sativum (garlic) on systolic and diastolic blood pressure in patients with essential hypertension. Pak J Pharm Sci 2013; 26(5):859-63. [ Google Scholar]
- Vazquez-Prieto MA, Rodriguez Lanzi C, Lembo C, Galmarini CR, Miatello RM. Garlic and onion attenuates vascular inflammation and oxidative stress in fructose-fed rats. J Nutr Metab 2011; 2011:475216. doi: 10.1155/2011/475216 [Crossref] [ Google Scholar]
- Ried K, Frank OR, Stocks NP. Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. Eur J Clin Nutr 2013; 67(1):64-70. doi: 10.1038/ejcn.2012.178 [Crossref] [ Google Scholar]
-
Ravichandra VD, Ramesh C, Swamy MK, Purushotham B, Rudramurthy GR. Anticancer plants: chemistry, pharmacology, and potential applications. In: Akhtar MS, Swamy MK, eds. Anticancer Plants: Properties and Application. Singapore: Springer; 2018. p. 485-515. 10.1007/978-981-10-8548-2_21
- Akinyemi AJ, Ademiluyi AO, Oboh G. Aqueous extracts of two varieties of ginger (Zingiber officinale) inhibit angiotensin I-converting enzyme, iron(II), and sodium nitroprusside-induced lipid peroxidation in the rat heart in vitro. J Med Food 2013; 16(7):641-6. doi: 10.1089/jmf.2012.0022 [Crossref] [ Google Scholar]
- Akinyemi AJ, Ademiluyi AO, Oboh G. Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger (Zingiber officinale) in rats fed a high cholesterol diet. J Med Food 2014; 17(3):317-23. doi: 10.1089/jmf.2012.0264 [Crossref] [ Google Scholar]
- Kim JH. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res 2012; 36(1):16-26. doi: 10.5142/jgr.2012.36.1.16 [Crossref] [ Google Scholar]
- Rhee MY, Cho B, Kim KI, Kim J, Kim MK, Lee EK. Blood pressure lowering effect of Korea ginseng derived ginseol K-g1. Am J Chin Med 2014; 42(3):605-18. doi: 10.1142/s0192415x14500396 [Crossref] [ Google Scholar]
- Jovanovski E, Bateman EA, Bhardwaj J, Fairgrieve C, Mucalo I, Jenkins AL. Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J Am Soc Hypertens 2014; 8(8):537-41. doi: 10.1016/j.jash.2014.04.004 [Crossref] [ Google Scholar]
- Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol 2015; 161:69-81. doi: 10.1016/j.jep.2014.09.049 [Crossref] [ Google Scholar]
- Wan X, Chen X, Liu L, Zhao Y, Huang WJ, Zhang Q. Berberine ameliorates chronic kidney injury caused by atherosclerotic renovascular disease through the suppression of NFκB signaling pathway in rats. PLoS One 2013; 8(3):e59794. doi: 10.1371/journal.pone.0059794 [Crossref] [ Google Scholar]
- Koo YE, Song J, Bae S. Use of plant and herb derived medicine for therapeutic usage in cardiology. Medicines (Basel) 2018; 5(2):38. doi: 10.3390/medicines5020038 [Crossref] [ Google Scholar]
-
Maulik SK, Banerjee SK. Uses of herbals in cardiac diseases: priority of evidence over belief. In: Mukherjee PK, ed. Evidence-Based Validation of Herbal Medicine. Boston: Elsevier; 2015. p. 515-29. 10.1016/b978-0-12-800874-4.00024-6
- Asher GN, Viera AJ, Weaver MA, Dominik R, Caughey M, Hinderliter AL. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement Altern Med 2012; 12:26. doi: 10.1186/1472-6882-12-26 [Crossref] [ Google Scholar]
- Larson AJ, Symons JD, Jalili T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr 2012; 3(1):39-46. doi: 10.3945/an.111.001271 [Crossref] [ Google Scholar]
- Ahmadipour B, Kalantar M, Hosseini SM, Yang LG, Kalantar MH, Abbas Raza SH. Hawthorn (Crataegus oxyacantha) extract in the drinking water of broilers on growth and incidence of pulmonary hypertension syndrome (PHS). Braz J Poult Sci 2017; 19(4):639-44. doi: 10.1590/1806-9061-2017-0558 [Crossref] [ Google Scholar]
- Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. J Res Med Sci 2014; 19(4):358-67. [ Google Scholar]
- Lobay D. Rauwolfia in the treatment of hypertension. Integr Med (Encinitas) 2015; 14(3):40-6. [ Google Scholar]
- Bahem R, Hoffmann A, Azonpi A, Caballero-George C, Vanderheyden P. Modulation of calcium signaling of angiotensin AT1, endothelin ETA, and ETB receptors by silibinin, quercetin, crocin, diallyl sulfides, and ginsenoside Rb1. Planta Med 2015; 81(8):670-8. doi: 10.1055/s-0034-1383408 [Crossref] [ Google Scholar]
- Sharma P, Sanadhya D. The king of bitters,”Andrographis paniculata”: a plant with multiple medicinal properties. J Plant Sci Res 2017; 33(1):117-25. [ Google Scholar]
- Awang K, Abdullah NH, Hadi AH, Fong YS. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J Biomed Biotechnol 2012; 2012:876458. doi: 10.1155/2012/876458 [Crossref] [ Google Scholar]
- Tanaka T, Homma Y, Kawamura Y. Kudzu (Pueraria lobata) vine isoflavone, puerarin, improves weight gain, glucose metabolism and osteoporosis and their biokinetics in ovariectomized mouse. Adv Obes Weight Manag Control 2017; 7(3):281-3. doi: 10.15406/aowmc.2017.07.00196 [Crossref] [ Google Scholar]
- Chen C, Chen C, Wang Z, Wang L, Yang L, Ding M. Puerarin induces mitochondria-dependent apoptosis in hypoxic human pulmonary arterial smooth muscle cells. PLoS One 2012; 7(3):e34181. doi: 10.1371/journal.pone.0034181 [Crossref] [ Google Scholar]
- Oh KS, Ryu SY, Oh BK, Seo HW, Kim YS, Lee BH. Antihypertensive, vasorelaxant, and antioxidant effect of root bark of Ulmus macrocarpa. Biol Pharm Bull 2008; 31(11):2090-6. doi: 10.1248/bpb.31.2090 [Crossref] [ Google Scholar]
- Devi RC, Sim SM, Ismail R. Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evid Based Complement Alternat Med 2012; 2012:539475. doi: 10.1155/2012/539475 [Crossref] [ Google Scholar]
- Francisco V, Costa G, Figueirinha A, Marques C, Pereira P, Miguel Neves B. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: contribution of chlorogenic acid. J Ethnopharmacol 2013; 148(1):126-34. doi: 10.1016/j.jep.2013.03.077 [Crossref] [ Google Scholar]
- Mishra R, Sharma S, Sharma RS, Singh S, Sardesai MM, Sharma S. Viscum articulatum Burm f aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells. J Ethnopharmacol 2018; 219:91-102. doi: 10.1016/j.jep.2018.03.005 [Crossref] [ Google Scholar]
- Kamohara S, Terasaki Y, Horikoshi I, Sunayama S. Safety of a Coleus forskohlii formulation in healthy volunteers. Personalized Medicine Universe 2015; 4:63-5. doi: 10.1016/j.pmu.2015.01.001 [Crossref] [ Google Scholar]
- Valls RM, Llauradó E, Fernández-Castillejo S, Puiggrós F, Solà R, Arola L. Effects of low molecular weight procyanidin rich extract from French maritime pine bark on cardiovascular disease risk factors in stage-1 hypertensive subjects: randomized, double-blind, crossover, placebo-controlled intervention trial. Phytomedicine 2016; 23(12):1451-61. doi: 10.1016/j.phymed.2016.08.007 [Crossref] [ Google Scholar]
- Carre G, Ouedraogo M, Magaud C, Carreyre H, Becq F, Bois P. Vasorelaxation induced by dodoneine is mediated by calcium channels blockade and carbonic anhydrase inhibition on vascular smooth muscle cells. J Ethnopharmacol 2015; 169:8-17. doi: 10.1016/j.jep.2015.03.037 [Crossref] [ Google Scholar]
- Veeramani C, Al-Numair KS, Chandramohan G, Alsaif MA, Pugalendi KV. Protective effect of Melothria maderaspatana leaf fraction on electrolytes, catecholamines, endothelial nitric oxide synthase and endothelin-1 peptide in uninephrectomized deoxycorticosterone acetate-salt hypertensive rats. J Nat Med 2012; 66(3):535-43. doi: 10.1007/s11418-011-0621-z [Crossref] [ Google Scholar]
- Aekthammarat D, Pannangpetch P, Tangsucharit P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine 2019; 54:9-16. doi: 10.1016/j.phymed.2018.10.023 [Crossref] [ Google Scholar]
- Brankovic S, Radenkovic M, Kitic D, Veljkovic S, Ivetic V, Pavlovic D. Comparison of the hypotensive and bradycardic activity of ginkgo, garlic, and onion extracts. Clin Exp Hypertens 2011; 33(2):95-9. doi: 10.3109/10641963.2010.531833 [Crossref] [ Google Scholar]
- Larson AJ, Symons JD, Jalili T. Quercetin: a treatment for hypertension? - a review of efficacy and mechanisms. Pharmaceuticals (Basel) 2010; 3(1):237-50. doi: 10.3390/ph3010237 [Crossref] [ Google Scholar]
- González-Peña D, Angulo J, Vallejo S, Colina-Coca C, de Ancos B, Sánchez-Ferrer CF. High-cholesterol diet enriched with onion affects endothelium-dependent relaxation and NADPH oxidase activity in mesenteric microvessels from Wistar rats. Nutr Metab (Lond) 2014; 11:57. doi: 10.1186/1743-7075-11-57 [Crossref] [ Google Scholar]
- Poh TF, Ng HK, Hoe SZ, Lam SK. Gynura procumbens causes vasodilation by inhibiting angiotensin II and enhancing bradykinin actions. J Cardiovasc Pharmacol 2013; 61(5):378-84. doi: 10.1097/FJC.0b013e31828685b3 [Crossref] [ Google Scholar]
- Ng HK, Poh TF, Lam SK, Hoe SZ. Potassium channel openers and prostacyclin play a crucial role in mediating the vasorelaxant activity of Gynura procumbens. BMC Complement Altern Med 2013; 13:188. doi: 10.1186/1472-6882-13-188 [Crossref] [ Google Scholar]
- Asgary S, Hashemi M, Goli-Malekabadi N, Keshvari M. The effects of acute consumption of pomegranate juice (Punica granatum L) on decrease of blood pressure, inflammation, and improvement of vascular function in patients with hypertension: a clinical trial. J Shahrekord Univ Med Sci 2015; 16(6):84-91. [ Google Scholar]
- de CC Pinto N, Campos LM, Evangelista ACS, Lemos ASO, Silva TP, Melo RCN. Antimicrobial Annona muricata L (soursop) extract targets the cell membranes of gram-positive and gram-negative bacteria. Ind Crops Prod 2017; 107:332-40. doi: 10.1016/j.indcrop.2017.05.054 [Crossref] [ Google Scholar]
- Moon SK, Kim SB, Kwon S, Cho SY, Park SU, Jung WS. Anti-hypertensive effect by single adminstration of Chunghyul-dan: a case series. J Korean Med 2018; 39(1):95-103. doi: 10.13048/jkm.18010 [Crossref] [ Google Scholar]
- Kook SH, Choi KC, Lee YH, Cho HK, Lee JC. Raphanus sativus L seeds prevent LPS-stimulated inflammatory response through negative regulation of the p38 MAPK-NF-κB pathway. Int Immunopharmacol 2014; 23(2):726-34. doi: 10.1016/j.intimp.2014.11.001 [Crossref] [ Google Scholar]
- Herrera-Arellano A, Miranda-Sánchez J, Avila-Castro P, Herrera-Alvarez S, Jiménez-Ferrer JE, Zamilpa A. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension A randomized, double-blind, lisinopril-controlled clinical trial. Planta Med 2007; 73(1):6-12. doi: 10.1055/s-2006-957065 [Crossref] [ Google Scholar]
- McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr 2010; 140(2):298-303. doi: 10.3945/jn.109.115097 [Crossref] [ Google Scholar]
- Hopkins AL, Lamm MG, Funk JL, Ritenbaugh C. Hibiscus sabdariffa L in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia 2013; 85:84-94. doi: 10.1016/j.fitote.2013.01.003 [Crossref] [ Google Scholar]
- Zhou D, Liu B, Xiao X, Dai P, Ma S, Huang W. The effect of safflower yellow on spinal cord ischemia reperfusion injury in rabbits. Oxid Med Cell Longev 2013; 2013:692302. doi: 10.1155/2013/692302 [Crossref] [ Google Scholar]
- Mokhtari-Zaer A, Khazdair MR, Boskabady MH. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J Phytomed 2015; 5(5):365-75. [ Google Scholar]
- El-Beshbishy HA, Hassan MH, Aly HA, Doghish AS, Alghaithy AA. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf 2012; 83:47-54. doi: 10.1016/j.ecoenv.2012.06.003 [Crossref] [ Google Scholar]
- Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 2010; 648(1-3):110-6. doi: 10.1016/j.ejphar.2010.09.003 [Crossref] [ Google Scholar]
- Mirmiran P, Bahadoran Z, Golzarand M, Rajab A, Azizi F. Ardeh (Sesamum indicum) could improve serum triglycerides and atherogenic lipid parameters in type 2 diabetic patients: a randomized clinical trial. Arch Iran Med 2013; 16(11):651-6. [ Google Scholar]
- da Cunha GH, de Moraes MO, Fechine FV, Frota Bezerra FA, Silveira ER, Canuto KM. Vasorelaxant and antihypertensive effects of methanolic fraction of the essential oil of Alpinia zerumbet. Vascul Pharmacol 2013; 58(5-6):337-45. doi: 10.1016/j.vph.2013.04.001 [Crossref] [ Google Scholar]
- Shen XC, Tao L, Li WK, Zhang YY, Luo H, Xia YY. Evidence-based antioxidant activity of the essential oil from Fructus A zerumbet on cultured human umbilical vein endothelial cells’ injury induced by ox-LDL. BMC Complement Altern Med 2012; 12:174. doi: 10.1186/1472-6882-12-174 [Crossref] [ Google Scholar]
- Maity S, Chatterjee S, Variyar PS, Sharma A, Adhikari S, Mazumder S. Evaluation of antioxidant activity and characterization of phenolic constituents of Phyllanthus amarus root. J Agric Food Chem 2013; 61(14):3443-50. doi: 10.1021/jf3046686 [Crossref] [ Google Scholar]
- Ardalani H, Hassanpour Moghadam M, Rahimi R, Soltani J, Mozayanimonfared A, Moradi M. Sumac as a novel adjunctive treatment in hypertension: a randomized, double-blind, placebo-controlled clinical trial. RSC Adv 2016; 6(14):11507-12. doi: 10.1039/c5ra22840a [Crossref] [ Google Scholar]
- Umar A, Imam G, Yimin W, Kerim P, Tohti I, Berké B. Antihypertensive effects of Ocimum basilicum L (OBL) on blood pressure in renovascular hypertensive rats. Hypertens Res 2010; 33(7):727-30. doi: 10.1038/hr.2010.64 [Crossref] [ Google Scholar]
- Olas B, Bryś M. Is it safe to use Acorus calamus as a source of promising bioactive compounds in prevention and treatment of cardiovascular diseases?. Chem Biol Interact 2018; 281:32-6. doi: 10.1016/j.cbi.2017.12.026 [Crossref] [ Google Scholar]
- Siddiqi HS, Mehmood MH, Rehman NU, Gilani AH. Studies on the antihypertensive and antidyslipidemic activities of Viola odorata leaves extract. Lipids Health Dis 2012; 11:6. doi: 10.1186/1476-511x-11-6 [Crossref] [ Google Scholar]
- Li D, Wang R, Huang J, Cai Q, Yang CS, Wan X. Effects and mechanisms of tea regulating blood pressure: evidences and promises. Nutrients 2019; 11(5):1115. doi: 10.3390/nu11051115 [Crossref] [ Google Scholar]
- Peng X, Zhou R, Wang B, Yu X, Yang X, Liu K. Effect of green tea consumption on blood pressure: a meta-analysis of 13 randomized controlled trials. Sci Rep 2014; 4:6251. doi: 10.1038/srep06251 [Crossref] [ Google Scholar]
- Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 2012; 32(6):421-7. doi: 10.1016/j.nutres.2012.05.007 [Crossref] [ Google Scholar]
- Hodgson JM, Puddey IB, Woodman RJ, Mulder TP, Fuchs D, Scott K. Effects of black tea on blood pressure: a randomized controlled trial. Arch Intern Med 2012; 172(2):186-8. doi: 10.1001/archinte.172.2.186 [Crossref] [ Google Scholar]
- Liu PL, Liu JT, Kuo HF, Chong IW, Hsieh CC. Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1β via heme oxygenase-1. Mediators Inflamm 2014; 2014:523684. doi: 10.1155/2014/523684 [Crossref] [ Google Scholar]
- Lee OH, Kim KI, Han CK, Kim YC, Hong HD. Effects of acidic polysaccharides from gastrodia rhizome on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed a high-fat diet. Int J Mol Sci 2012; 13(1):698-709. doi: 10.3390/ijms13010698 [Crossref] [ Google Scholar]
- Liu Y, Wang G, Yang M, Chen H, Zhao Y, Yang S. Arctigenin reduces blood pressure by modulation of nitric oxide synthase and NADPH oxidase expression in spontaneously hypertensive rats. Biochem Biophys Res Commun 2015; 468(4):837-42. doi: 10.1016/j.bbrc.2015.11.041 [Crossref] [ Google Scholar]
- Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc Drugs Ther 2009; 23(2):145-51. doi: 10.1007/s10557-008-6155-2 [Crossref] [ Google Scholar]
- Monteiro FS, Silva AC, Martins IR, Correia AC, Basílio IJ, Agra MF. Vasorelaxant action of the total alkaloid fraction obtained from Solanum paludosum Moric (Solanaceae) involves NO/cGMP/PKG pathway and potassium channels. J Ethnopharmacol 2012; 141(3):895-900. doi: 10.1016/j.jep.2012.03.032 [Crossref] [ Google Scholar]
- Dolati S, Ahmadi M, Rikhtegar R, Babaloo Z, Ayromlou H, Aghebati-Maleki L. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int Immunopharmacol 2018; 61:74-81. doi: 10.1016/j.intimp.2018.05.018 [Crossref] [ Google Scholar]
- Leong XF. The spice for hypertension: protective role of Curcuma longa. Biomed Pharmacol J 2018; 11(4):1829-40. doi: 10.13005/bpj/1555 [Crossref] [ Google Scholar]
- Owolabi OJ, Ayinde BA, Nworgu ZA, Ogbonna OO. Antidiarrheal evaluation of the ethanol extract of Musanga cecropioides stem bark. Methods Find Exp Clin Pharmacol 2010; 32(6):407-11. doi: 10.1358/mf.2010.32.6.1440745 [Crossref] [ Google Scholar]
- Chaudhari HS, Bhandari U, Khanna G. Preventive effect of embelin from Embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med 2012; 78(7):651-7. doi: 10.1055/s-0031-1298379 [Crossref] [ Google Scholar]
- Yousefi K, Fathiazad F, Soraya H, Rameshrad M, Maleki-Dizaji N, Garjani A. Marrubium vulgare L methanolic extract inhibits inflammatory response and prevents cardiomyocyte fibrosis in isoproterenol-induced acute myocardial infarction in rats. Bioimpacts 2014; 4(1):21-7. doi: 10.5681/bi.2014.001 [Crossref] [ Google Scholar]
- Zarshenas MM, Zargaran A. A review on the Avicenna’s contribution to the field of cardiology. Int J Cardiol 2015; 182:237-41. doi: 10.1016/j.ijcard.2014.12.145 [Crossref] [ Google Scholar]
- Zargaran A. Ancient Persian medical views on the heart and blood in the Sassanid era (224-637 AD). Int J Cardiol 2014; 172(2):307-12. doi: 10.1016/j.ijcard.2014.01.035 [Crossref] [ Google Scholar]
- Mohammadinasab R, Ghazi Sha’rbaf J, Taheri-Targhi S. Letter to the Editor Regarding “A Note About the Ancestral Origin of Abu Al Husain Ibn Abdullah Ibn Sina, Avicenna (980-1037 CE)”. World Neurosurg 2020; 136:431. doi: 10.1016/j.wneu.2020.01.053 [Crossref] [ Google Scholar]
- Taghizadieh A, Mohammadinasab R, Ghazi Sha’rbaf J, Safiri S. The first description of asthma due to heart conditions in the history of medicine. Chest 2020; 158(2):461-3. doi: 10.1016/j.chest.2020.01.011 [Crossref] [ Google Scholar]
- Gholami-Ahangaran M, Bahmani M, Zia-Jahromi N. Comparative and evaluation of anti-leech (Limnatis nilotica) effect of olive (Olea europaea L) with levamisol and tiabendazole. Asian Pac J Trop Dis 2012; 2 Suppl 1:S101-S3. doi: 10.1016/S2222-1808(12)60132-7 [Crossref] [ Google Scholar]
- Mardani-Nejhad S, Vazirpour M. Ethno-botany of medicinal plants by Mobarakeh’s people (Isfahan). Journal of Herbal Drugs 2012; 3(2):111-26. [ Google Scholar]
- Baharvand-Ahmadi B, Bahmani M, Tajeddini P, Rafieian-Kopaei M, Naghdi N. An ethnobotanical study of medicinal plants administered for the treatment of hypertension. J Renal Inj Prev 2016; 5(3):123-8. doi: 10.15171/jrip.2016.26 [Crossref] [ Google Scholar]
- Khodayari H, Amani SH, Amiri H. Ethnobotanical study of medicinal plants in different regions of Khuzestan province. Eco-phytochemical Journal of Medicinal Plants 2015; 2(4):12-26. [ Google Scholar]
- Bahmani M, Asadi-Samani M. A short look to the most important medicinal plants effective on wound healing. Journal of Injury and Inflammation 2016; 1(2):e07. [ Google Scholar]
- Moradi MT, Asadi-Samani M, Bahmani M. Hypotensive medicinal plants according to ethnobotanical evidence of Iran: a systematic review. Int J Pharmtech Res 2016; 9(5):416-26. [ Google Scholar]
- Ghasemi Pirbalouti A, Momeni M, Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. Afr J Tradit Complement Altern Med 2013; 10(2):368-85. doi: 10.4314/ajtcam.v10i2.24 [Crossref] [ Google Scholar]
- Darvish Damavandi R, Shidfar F, Rajab A, Mohammadi V, Hosseini S. The effects of cashew consumption on serum glucose, insulin and lipoprotein in type 2 diabetic patients. Iran J Endocrinol Metab 2012; 14(4):325-34. [ Google Scholar]
- Delfan B, Saki K, Bahmani M, Rangsaz N, Delfan M, Mohseni N. A study on anti-diabetic and anti-hypertension herbs used in Lorestan province, Iran. J Herbmed Pharmacol 2014; 3(2):71-6. [ Google Scholar]
- Baharvand-Ahmadi B, Asadi-Samani M. A mini-review on the most important effective medicinal plants to treat hypertension in ethnobotanical evidence of Iran. J Nephropharmacol 2017; 6(1):3-8. [ Google Scholar]