Advanced pharmaceutical bulletin. 13(3):512-520.
doi: 10.34172/apb.2023.062
Mini Review
Drug Delivery of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) to Target Brain Tumors
Soheil Mehrdadi * 
Author information:
Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Padua, Italy
Abstract
Brain, predisposed to local and metastasized tumors, has always been the focus of oncological studies. Glioblastoma multiforme (GBM), the most common invasive primary tumor of the brain, is responsible for 4% of all cancer-related deaths worldwide. Despite novel technologies, the average survival rate is 2 years. Physiological barriers such as blood-brain barrier (BBB) prevent drug molecules penetration into brain. Most of the pharmaceuticals present in the market cannot infiltrate BBB to have their maximum efficacy and this in turn imposes a major challenge. This mini review discusses GBM and physiological and biological barriers for anticancer drug delivery, challenges for drug delivery across BBB, drug delivery strategies focusing on SLNs and NLCs and their medical applications in on-going clinical trials. Numerous nanomedicines with various characteristics have been introduced in the last decades to overcome the delivery challenge. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) were introduced as oral drug delivery nanomedicines which can be encapsulated by both hydrophilic and lipophilic pharmaceutical compounds. Their biocompatibility, biodegradability, lower toxicity and side effects, enhanced bioavailability, solubility and permeability, prolonged half-life and stability and finally tissue-targeted drug delivery makes them unique among all.
Keywords: Brain tumors, Glioblastoma multiforme, Blood-brain barrier, Solid lipid nanoparticles, Nanostructured lipid carriers, Brain drug delivery
Copyright and License Information
©2023 The Authors.
This is an Open Access article distributed under the terms of the Creative Commons Attribution (CC BY), which permits unrestricted use, distribution, and reproduction in any medium, as long as the original authors and source are cited. No permission is required from the authors or the publishers.
Introduction
Cancers, the second cause of global mortality, are accounted for seven to nine million deaths from 2005 to 2015 (Figure 1).1 Despite significant progress in diagnosis, treatment and prevention studies of cancer in the last decades, the mortality rate of most cancer cases, especially brain tumors, is still considerable and remains a medical challenge.
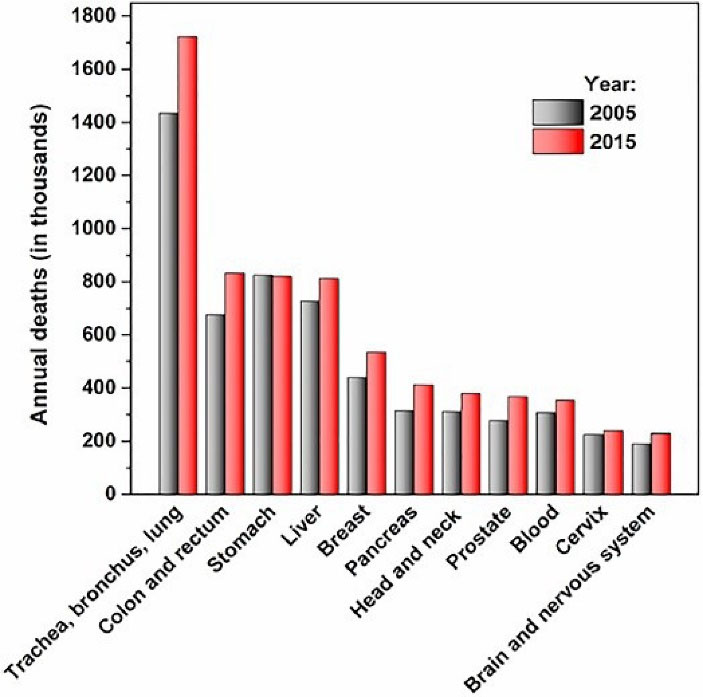
Figure 1.
Cancer world demography (2005-2015)
.
Cancer world demography (2005-2015)
Glioblastoma multiforme (GBM), the most invasive and common malignant tumor of brain (55% of all cases),2 has two types: primary (90%) and secondary (10%): the former develops acutely with no previous lower grade pathological signs or symptoms, while the latter which is seen mostly at younger ages arises from evolving and transforming astrocytomas. Both have different genetic profiles and are detectable by specific cell markers, although clinically similar.3 Surgical excision of the tumor, co-temporaneous post-surgical radiotherapy and chemotherapy are the therapeutic regimen. Nevertheless, the survival rate is still 1 to 2 years (3–5%).4
The abovementioned therapeutic regimen does not offer a practical solution to target brain tumors in an effective way which can be justified due to the failure of anticancer drugs to exert their maximum effect mainly due to the following: (a) overexpression of P-glycoprotein (P-gp) receptors in GBM chemoresistant cancer cells causing increased drug efflux; (b) hypoxic tumor tissues further aggravating drug delivery by promoting ischemia; (c) high heterogeneity and variability of GBM at molecular, histopathological and genetic levels5,6; (d) the effect of treatment on cancer tissue is unpredictable7; and (e) the intrinsic feature of GBM; sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, enabling replicative immortality, inducing angiogenesis, resisting cell death, evading immune destruction and reprogramming cellular energetics. The highly-resistant character of GBM cancer cells makes them relapse and penetrate healthy brain tissues quickly resulting from their unique cellular heterogeneity, presenting a challenging case in cancer patient management.
Searching for a brain tumor targeted drug delivery system, numerous “nanomedicines” and de-novo chemotherapeutics have been investigated to overcome low drug penetration and cellular resistance,4 and to specifically and accurately deliver the drug to their target tissue without having adverse effects on the adjacent intact tissues.8 Despite promising in-vitro results, most in-vivo studies fail when administered systemically mainly due to the biological and pathological barriers, namely as blood-brain barrier (BBB), blood-brain tumor barrier (BBTB), arachnoid barrier and blood cerebrospinal fluid barrier (BCSF) barrier, which restrict the delivery of chemotherapeutic drugs to the tumor tissue.9 Hence, it is necessary to investigate these more profoundly.
The focus of the present mini review is on two types of drug delivery systems, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), to target brain tumors. But, it is worthwhile to discuss first the barriers for brain anticancer drug delivery.
Barriers to brain drug delivery
Blood-brain barrier
BBB by modulating the transfer of different substances from the blood to CNS maintains a stable concentration of endogenous and exogenous substances in the body which are selected according to their structural and molecular features.10 BBB originates from the neurovascular unit which is extended along the cerebral capillaries, and is formed by endothelial cells, pericytes, vascular smooth muscle cells, neurons, astrocytes and perivascular macrophages.11 However, there are anatomical differences between brain and peripheral capillaries; the peripheral capillaries lack fenestrations and intercellular pores (while present in brain capillaries) because of endothelial tight and adherent junctions which limit transportation through paracellular pathways. Another difference is related to their components; tight junctions are composed of cingulin, occluding, claudins and zonula occludens proteins, while adherens junctions are cadherins.11 Different molecules (e.g. nutrients, enzymes and proteins) are transferred inside and outside of the CNS mediated by four mechanisms (Table 1). The unique structure of BBB restricts the penetration of almost 98% of new therapeutic molecules into the brain tissue following systemic administration which poses a serious problem to access the brain microvasculature (20 m2 surface area, 640 km length).12 This large and long vascularization provides a rapid transportation (by simple diffusion) of tiny molecules less than a second,13 which is almost impermeable through the peripheral circulation.13 Furthermore, periendothelial accessory structures (astrocytes) act to mediate and produce growth factors and cytokines, keep potassium ion levels and inactivate neurotransmitters.14
Table 1.
Different mechanisms of physiological transport across BBB
|
Pathway
|
Transporter
|
Process
|
Feature of molecules
|
Note
|
Reference
|
| Paracellular diffusion |
Across cells |
Non-saturable, non-competitive |
Small water-soluble |
Limited diffusion due to endothelial cells tight junction |
15
|
| Transcellular diffusion |
Across cells |
Non-saturable, non-competitive |
High lipophilicity, unfit for active efflux transport, low molecular weight ( < 400 Dalton) |
- |
15,16
|
| Carrier- mediated transport |
Glucose transporters (GLUT1 & GLUT3), monocarboxylate (lactate; pyruvate) transporter system (MCT1), specialized carriers (essential amino acids and vitamins) |
Active transport by selective membrane- bound carrier systems |
Glucose, galactose, amino acids, nucleosides, lactates and pyruvates, adenine and guanine, choline, vitamins and hormones |
Establishment of transient narrow pores |
15,17-19
|
| Receptor- mediated endocytosis |
Specific receptors on the luminal side of the barrier for insulin,
insulin-like growth factors, angiotensin II, folates and transferrin |
Absorptive
transcytosis without specific plasma- membrane receptors |
Endogenous proteins
and hormones, low- density lipoproteins (LDL), Polycationic proteins (albumins and immunoglobulins) |
Polycationic substances bound to
negative charges of endothelial cells of plasma membrane |
15,20,21
|
The different “drug delivery” transport mechanisms across BBB have the principal role of delivering drugs, considering that pro-drugs and drugs, resembling endogenous compounds and nutrients, are encapsulated according to the required therapeutic dosage of compounds and nutrients.13
Besides, it is proved that the P-gp receptors (an ATP-dependent drug transport protein) on the BBB endothelial cells interfere with the penetration of molecules inside by exporting them outside the cells. Overcoming P-gp drug export mechanism could maximize massively the drug absorption into the brain tissue.22,23
Blood-brain tumor barrier
In definition, when a brain tumor is formed, the BBB will be based between this new tumor tissue and capillary vessels. Thus, the BBB is referred to as the BBTB.24 According to the shape and permeability of the BBTB, there are three stages which mainly rely on the progression of adjacent brain tumor changes.24
In stage one (early-stage malignant tumor), the BBTB capillaries are consistent, non-fenestrated and intact, consequently the normal brain capillaries are still being supplied with necessary growth nutrients.25 However, during stage two which is tumor growth, after the adjacent intact brain tissues are invaded by cancer cells and the tumor tissue enlarges more than 2 mm, new capillaries form by the angiogenesis mechanism (neovasculature) which are consistent with fenestrations of about 12 nm. This in turn changes the permeability and subsequently only molecules smaller than twelve nm can infiltrate inside BBTB.25,26 In stage three, inter-endothelial gaps are established between cerebral endothelial cells (CECs) due to continuous tumor growth which consequently damage the BBTB integrity.
A study in mouse reported thinner than normal microvessel basement membrane of CECs with 1 μm inter-endothelial gaps and 48 nm fenestration size.25 The high permeability of BBTB can be used to accumulate higher doses of anticancer drugs in the tumor site.27
The damaged BBTB forms tiny holes to compensate for their high metabolic demands and this phenomenon is widely seen in GBM. Hypoxia triggers the angiogenesis in particular zones of GBM and ultimately damages the BBB integrity.28 Although, GBM spread aggressively and rapidly in the adjacent intact tissues this does not affect the BBTB with enhanced permeability and retention. Therefore, overcoming the BBTB promotes higher anticancer drug delivery to GBM tumors.29,30
Tumor microenvironment
In order to have a more efficient drug delivery and successful treatment of brain cancers, a complete studying of tumor microenvironment (TME) is also necessary to interpret and justify the tumor molecular and biological processes.31,32 Generally, TME is considered as a complex heterogeneous environment where different physiological factors, such as mechanical stresses and protein-binding drug degradation, is required to be evaluated for a drug delivery system. So far, various components of TME have been studied and explained, namely as extracellular matrix (microglia, astrocytes and neurons) and blood vessels (also the ones forming the BBB), through the latter the vital role of immune and lymphatic systems and vascular cells in successful brain tumor treatment has been proved.33-38
Macrophages of the myeloid cells and microglia – collectively referred to as tumor-associated macrophages (TAMs)39,40 – have been provoked by GBM cells to generate immunosuppressive tumor- related cytokines and elevated T-cell apoptosis.41 Moreover, GBM cells also inhibit antitumor immune system activities by preventing immune-stimulatory cytokines’ production and activating the regulatory T-cells.42 Further studies suggest that preventing TAMs activity and readjusting them phenotypically to be functional against tumor progression is more efficacious than deactivating them,32,43 and this concept has been the basis of the innovation of the recently introduced anti-cancer therapies; immune-checkpoint inhibitors, cellular therapies (such as chimeric antigen receptor T cells) and vaccines.42-44
Nevertheless, unlike their promising features as a new approach in cancer therapy,31 there are factors hampering their maximum therapeutic effect in tumor tissue, the most important one is the BBB and its contribution toward immunotherapies penetration and other chemotherapeutics (such as Temozolomide) local co-existence. It is commonly believed that tumor vessels are significantly more permeable than the ones in intact tissues,45 and could diffuse more blood from vessels into the interstitial space to increase the interstitial fluid pressure.
The tumor vessels lose their well-established structure with the progression of tumor; they are not as functional as arteries, capillaries and veins in intact tissues. Decreased blood velocity and pressure gradient,46,47 increased interstitial fluid pressure and suppressed transvascular convection prevent drug delivery to the cancer tissue and subsequently limits the drug diffusion.48,49 Furthermore, the systemic administration diminishes even more delivery of chemotherapeutic by exposing them to enzymatic/hydrolytic degradation. Hence, only small doses reach the cancer site with hypoxia and low pH which further aggravate the situation and the administered drug will not exert a therapeutic effect.
Challenges for oral delivery of anticancer drugs across BBB
The oral route to target brain cancers is a striking challenge, in part due to the limited oral absorption, short plasma half-life and the BBB as a barrier. The oral delivery of hydrophilic drugs to brain requires absorption and infiltration through the gastrointestinal tract (GIT) and then across the BBB. Moreover, the hydrogen bonds formed between hydrophilic drugs and GIT aqueous contents, hindering the absorption, further limits epithelial infiltration process.50 Additionally, peptide drugs are rapidly degraded upon their administration in GIT and this decreases their plasma half-lives.51
The application of nanomedicines for brain anticancer drug delivery
During recent decades, various nanomedicines of different size, material (Synthetic, natural, organic and inorganic) and shape have been employed for cancer treatment,52,53 and these components of construction affect their characteristics. Since their early introduction they have been rapidly evolved, mostly due to replacing unfit conventional clinical techniques with improved ones for refractory diseases, and since then have found their application in pharmaceutical and biomedical industries.54
In order to exert their maximum therapeutic effect with the lowest side effects, effective nanomedicines must be precisely engineered to target the complex pathophysiology of each disease. Therefore, they could be modified in such a way to be responsive according to different endogenous or exogenous stimuli; this feature can be employed for controlled and sustained release of loaded drug. Besides their application for therapeutic purposes, some of them (e.g. inorganic and polymeric/lipid nanoparticles) have been employed for diagnosis purposes. Their dual-purpose application both as therapeutic and diagnostic resulted in the introduction of “theranostic” nanoparticles for which, among all, only synthetic polymers are used.
Each disease possesses its own specific etiology which is also influenced highly by environmental and genetic factors, and all these differences apply a very specific drug delivery design for targeting the affected area with minimum adverse effect on adjacent intact tissues resulting in tumor regression or complete healing. With regard to the CNS diseases, they all share one challenge: drug delivery through the BBB. In order to overcome this issue, a huge range of therapeutics such as drugs (functionalized with specific targeting segments), DNA/RNA, genes, enzymes, antioxidants can be precisely loaded into the matrix of polymeric/lipid nanomedicines. In some studies on brain cancer, the majority of synthetic nanomedicines used are polymers such as polyethylene glycol (PEG) or Poly(lactide-co-glycolide) with different surface/physicochemical properties, size and shape.55,56 however, their degradation produces acidic by-products which are toxic for brain tissue, making them unsuitable for extended drug delivery application, hence, inorganic nanoparticles (still toxic) and lipid-based structures have been introduced.
With regard to brain cancers, drug delivery happens at local or systemic level; the former will permeate the BBB, accumulating a higher concentration of drug with minimum adverse effects for adjacent intact tissues, evading also degradation/hydrolysis and clearance processes before its in-site delivery. Implants, intraventricular/intrathecal and convection enhanced delivery (CED) all can be named as examples of local drug delivery. The latter, systemic drug delivery on the other hand, is the most common one in terms of compliance for patients since it doesn’t require surgical or clinical intervention, with possibility of repeated doses which makes them less invasive and more favorable compared to the local drug delivery.
So far, numerous nanomedicines with various size, surface charge and hydration and targeting character have been exploited for brain anticancer delivery, among them nanoparticles, nanofibers and hydrogels (Polymer-based nanostructures), lipid nanocapsules, liposomes, SLNs and NLCs (lipid-based nanostructures) have been widely studied.
SLNs and NLCs belong to lipid-based nanosystems of drug delivery. They offer better advantages over the polymeric or inorganic nanoparticles; biocompatibility, better penetration through the BBB without any structural modification influencing their function and easy large-scale production. Nevertheless, their low loading capacity is a disadvantage for their clinical application hence the low number of systems marketed commercially.57
Solid lipid nanoparticles
Seeking for a substitute nanostructured system with lower toxicity, higher loading capacity and stability SLNs were developed and introduced thirty years ago,58 and since then numerous studies have proved their efficacy and advantage over emulsions, micelles, polymeric nanoparticles and liposomes.59
SLNs are composed of different lipids with the same features: containing surfactants/co-surfactants, solidness at various temperatures and low melting points. As for lipids cetyl palmitate, Compritol® 888 ATO, Precirol® ATO5, glycerol monostearate, stearic acid, stearyl alcohol, and for surfactants (function also as a stabilizer) dimethyl dioctadecyl ammonium bromide and Tween® 80 and poloxamer 188 have been used as the most common ones. The appropriate choice of lipids, surfactants and the composition of SLNs (the solid core: 0.1–30% w/w, surfactants: 0.5–5% w/v) influences their release profile, drug encapsulation, stability over time, surface charge, polydispersity, size and physicochemical features.
SLNs have several advantages; (a) ability to effectively deliver both hydrophilic and lipophilic drugs to various tissues, (b) potential encapsulation with a wide range of therapeutic molecules, such as oligonucleotides, peptides, genes and other tiny nanoparticles like superparamagnetic iron oxide particles, (c) ability to protect the loaded therapeutic molecule from reticuloendothelial system clearance, (d) poor water solubility that favors the encapsulated substance for controlled and sustained release, (e) long-term stability and lower toxicity making them applicable for long-term administration, (f) due to their biocompatibility, they are easily sterilized and there is no need for organic solvents use which might influence the toxicity of the final product, (g) they have large-scale industrial production capacity, (h) with modified targeting features they can specifically target the affected tissue.
Their disadvantages can be addressed as the following; a) encapsulated therapeutic particles export, (b) gelation predisposition, and (c) low encapsulation efficiency.58,59 The latter is the result of the crystallization process which leaves the lipid core internal structure without enough space for therapeutic substance loading.
Nanostructured lipid carriers
NLCs were introduced by Müller et al60 to improve the low-encapsulation efficiency of SLNs, with increased internal free space in solid lipid core structures. To synthesize such structure, a mixture of liquid and solid lipids with mono-, di- and triglycerides of different chain lengths is employed.61 Besides improving the encapsulation efficiency, other drawbacks of SLNS were improved, namely as stability and no drug expulsion during storage.61,62
It has been proved that hydrophobic drugs have higher dissolution rate in the liquid lipids than in solid ones, leading to increased encapsulation efficiency and higher solubility of drugs which in turn even results in higher encapsulation efficiencies.63 However, for hydrophilic drugs a lipid conjugation approach is used, by which the functional group of the drug (e.g. amine group) is conjugated with the functional group (e.g. carboxylic acid group) of lipids like oleic acid, through carbodiimide or another type of chemistry.
Among all the liquid lipids in use for NLCs Capmul® MCM C8, L-phosphatidyl choline (PC), Tegosoft® M, Tegosoft® P, soy lecithin, sesame oil, Speziol® EOL NF, Mygliol® 812 N, almond oil, olive oil, Suppocire® NC, cetiol, peanut oil, corn oil, soybean oil and oleic acid can be named. As for the surfactants N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethyl-ammonium chloride (DOTMA), Tween® 20, Tween® 80, Lutrol F68, Tego Care 450, Pluronic® F68, Speziol® TPGS Pharma, MyrjTM 59, Span® 85, Eumulgin SML, Cremophor® RH, Cremophor® EL. For NLCs synthesis the following ratio could be employed: the surfactant concentration 0.25–6% w/v, solid and liquid lipid 4:1–1:4, total lipid concentration 1–30% w/v.
Novel drug delivery strategies for brain tumors
Targeting moieties
The inability to locate or target cancer tissue; deposition of drug in the wrong tissue; Adsorption of drug by intact cells; Low in-site amount of therapeutic nanoparticles following poor cell endocytosis have been mentioned as the main reasons of failure in cancer targeted drug delivery of new biotechnological medicines.
Hence, exploiting cell and molecular biology techniques, specific targeting moieties for GBM have been discovered which have been under investigation for therapeutic purposes by nanotechnology. There are two ways to target tumor cells by nanoparticles; passive in which the tiny therapeutic molecules of drug reach and accumulate in cancer tissue by taking advantage of extravasation process through the “leaky” vessels, and active in which specific ligands attach to a specific receptor or molecular marker of the cancer cell which this feature in turn can be used for targeted-drug delivery.64 Since then, a new concept called ”biosensors” has been emerged in nanotechnology and drug delivery systems,65 and there have been clinical trials to investigate this concept for active drug delivery (Table 2).
|
Clinicaltrials ID
|
Condition
|
Intervention
|
Phase
|
| NCT00734682 |
Glioblastoma, gliosarcoma, anaplastic astrocytoma, anaplastic oligodendroglioma |
Drug: Nanoliposomal CPT- 11 |
Phase 1 |
| NCT02340156 |
Recurrent glioblastoma |
Genetic: SGT-53
Drug: Temozolomide |
Phase 2 |
| NCT00769093 |
Brain neoplasms |
Drug: Ferumoxytol |
Phase 1 |
| NCT00313599 |
CNS tumor |
Drug: lapatinib, paclitaxel |
Phase 1 |
| NCT01967810 |
Glioma glioblastoma
brain tumor, recurrent |
Drug: ANG1005
Drug: Bevacizumab |
Phase 2/ ongoing |
| NCT02048059 |
Breast cancer brain metastases |
Drug: ANG1005 |
Phase 2/ ongoing |
| NCT02820454 |
Brain metastases |
Drug: AGuIX Radiation: whole brain
radiation therapy |
Phase 1/ongoing |
| NCT03020017 |
Gliosarcoma, recurrent GBM |
NU-0129, spherical nucleic acid (SNA) arranged on the surface of a small spherical gold nanoparticle/ targeted molecular therapy |
Early Phase 1/on going |
| NCT02766699 |
GBM, astrocytoma, grade IV |
EGFR(V)-EDV-Dox [EGFR
(vectibix sequence) targeted EnGeneIC dream vectors containing doxorubicin] |
Phase 1/ongoing |
| NCT03086616 |
Diffuse intrinsic pontine glioma |
CED of nanoliposomal
irinotecan (nal-IRI) |
Phase 1/ongoing |
| NCT02022644 |
High grade glioma |
CED of nanoliposomal
irinotecan |
Phase 1/ongoing |
| NCT01386580 |
Brain metastases,
malignant glioma |
2B3-101 (phase1)
/Trastuzumab (phase2) |
Phase1&2/completed
(2014) |
| NCT02861222 |
Malignant glioma |
Liposomal doxorubicin
(MYOCET) |
Phase 1/completed
(2013) |
| NCT00944801 |
GBM |
PEGylated liposomal
doxorubicin |
Phase1&2/completed
(2009) |
| NCT00019630 |
Brain tumor |
Doxorubicin HCl liposome |
Phase 1/completed |
| NCT01906385 |
GBM, astrocytoma |
Rhenium nanoliposome |
Phase 1&2/ongoing |
| NCT01517464 |
Neoplasm |
Genetic: SGT-94 Liposome |
Phase 1/ongoing |
| NCT01266096 |
Malignant brain tumors |
Drug: PET scan with 124I- cRGDY-PEG-dots silica nanoparticles |
Phase 0/ongoing |
| NCT03020017 |
Gliosarcoma recurrent glioblastoma |
Laboratory Biomarker Analysis Pharmacological Study
Drug: Targeted Molecular Therapy
gold nanoparticles |
Early Phase1/ongoing |
Cancer cells overexpress numerous surface receptors which can be targeted by various biological ligand and moieties in drug delivery for brain cancer treatment; so far protein, peptides and antibodies have been under investigation. According to some studies (Table 3), there are various endothelial cell receptors in the brain for which there are specific ligands that can be exploited for the drug delivery purposes through the BBB. Nevertheless, given their ubiquitous expression in body cells, the risk of non-specific adverse effects might limit this approach.
Table 3.
Targeting moieties for brain drug delivery of nanomedicines
|
Class of ligand
|
Model drug
|
Receptor
|
Nanoparticle
|
Reference
|
| Antibodies |
Loperamide |
Transferrin/anti-transferrin receptor monoclonal antibodies (OX26 or R17217) |
human serum albumin (NHS-PEG-MAL-5000 linker) |
66
|
| Antibodies |
_ |
Transferrin |
Transferrin/bovine serum albumin |
67
|
| Antibodies |
Loperamide |
Insulin/anti-insulin receptor monoclonal antibody (29B4) |
human serum albumin (NHS-PEGMAL-5000 linker) |
68
|
| Antibodies |
Anti-IL13αR mAB |
IL3αR2 |
_ |
69,70
|
| Antibodies |
Specific mAB |
CD133 |
Carbon Nanotubes |
71
|
| Peptides |
paclitaxel |
Angiopep (Thr-Phe-Phe-Tyr- Gly-Gly-Ser-Arg-Gly-Lys-Arg- Asn-Asn-Phe-Lys-Thr-Glu-Glu-Tyr)/ low density lipoprotein |
Angiopep-conjugated poly(ethylene glycol)-co- poly(ε-caprolactone) copolymer |
72
|
| Peptides |
loperamide |
H-2N-Gly-l-Phe-d-Thr-Gly-l-Phe-l-Leu-l-Ser-O-ß-d- glucose-CONH2 |
Gly-l-Phe-d-Thr-Gly-l-Phe-l-Leu-l-Ser(O-β-d-glucose)- CONH2 bound to poly(D,L- lactide-co-glycolide) |
73
|
| Peptides |
Synthetic RGD |
αvβ3 integerin |
_ |
74,75
|
| Peptides |
Tat protein (from HIV) |
_ |
liposomes |
76
|
| Proteins |
siRNA |
Transferrin |
Cyclodextrin polymer-based |
77
|
| Proteins |
Chlorotoxin (from scorpion) |
Membrane bound matrix metalloproteinase-2 (MMP-2) |
- |
78
|
Intranasal drug delivery; a “shortcut” to brain
Intranasal delivery is a novel therapeutic drug delivery directly to the brain through the epithelium of the olfactory nerve (cranial nerve І) and trigeminal nerve (cranial nerve V) as anatomic connections. Since the nanoparticles are administered in nasal cavity, they protect the chemotherapeutic drug from the biophysical barriers namely as BBB. However, the administered drug is not able to recognize the intact and cancer brain tissue.79 There have been studies of successful brain delivery of anticancer drugs (such as methotrexate, 5-fluorouracil and raltitrexed) using intranasal delivery, evaluating the cellular mechanisms, involved cellular receptor and main vectors.80 Given its novelty, still further studies are required to assess the benefits and drawback of this route for brain cancer treatment.
Conclusion
Despite promising progress in recognizing pathophysiological and cellular behavior of malignant brain tumors such as GBM, yet they remain a medical challenge with high mortality rate. Recent in-depth understanding of the biochemistry, specific markers, ligands and receptors involved resulted in novel chemotherapeutic drugs, which are mostly hydrophobic and consequently reach into insufficiently to the tumor tissue to exert their maximum therapeutic effect. Furthermore, unique structure of BBB and BBTB which function as a barrier prevent in-site anticancer drug delivery to the cancer tissue. Hence, various nanomedicines have been investigated with in-vitro/in-vivo studies to overcome these barriers to provide high drug diffusion, controlled drug release profile, tumor-specific targeting and long-term blood circulation. Lipid nanoparticles among all offer promising drug delivery and can be modified in such a way to by-pass barriers and deliver the encapsulated therapeutics to the affected brain tissue. They also offer so many advantages over other polymeric nanoparticles with enhanced efficacy, reduced toxicity and enhanced drug stability.
Competing Interests
The author declares no conflict of interest.
Ethical Approval
There is none to be disclosed.
References
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388(10053):1459-544. doi: 10.1016/s0140-6736(16)31012-1 [Crossref] [ Google Scholar]
- Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets 2009; 13(6):701-18. doi: 10.1517/14728220902942348 [Crossref] [ Google Scholar]
- Bastiancich C, Danhier P, Préat V, Danhier F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J Control Release 2016; 243:29-42. doi: 10.1016/j.jconrel.2016.09.034 [Crossref] [ Google Scholar]
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 2009; 18(8):1061-83. doi: 10.1517/13543780903052764 [Crossref] [ Google Scholar]
- Ulrich TA. Cellular Mechanobiology of Glioblastoma Multiforme. Berkeley: University of California; 2011.
- Jue TR, McDonald KL. The challenges associated with molecular targeted therapies for glioblastoma. J Neurooncol 2016; 127(3):427-34. doi: 10.1007/s11060-016-2080-6 [Crossref] [ Google Scholar]
- Parker NR, Hudson AL, Khong P, Parkinson JF, Dwight T, Ikin RJ. Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci Rep 2016; 6:22477. doi: 10.1038/srep22477 [Crossref] [ Google Scholar]
- Bragagni M, Mennini N, Ghelardini C, Mura P. Development and characterization of niosomal formulations of doxorubicin aimed at brain targeting. J Pharm Pharm Sci 2012; 15(1):184-96. doi: 10.18433/j3230m [Crossref] [ Google Scholar]
- Ying X, Wen H, Lu WL, Du J, Guo J, Tian W. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release 2010; 141(2):183-92. doi: 10.1016/j.jconrel.2009.09.020 [Crossref] [ Google Scholar]
- Mehrdadi S. Acute bacterial meningitis: diagnosis, treatment and prevention. J Arch Mil Med 2018; 6(4):e84749. doi: 10.5812/jamm.84749 [Crossref] [ Google Scholar]
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 2010; 64(2):328-63. doi: 10.1016/j.brainresrev.2010.05.003 [Crossref] [ Google Scholar]
- Pardridge WM. Why is the global CNS pharmaceutical market so under-penetrated?. Drug Discov Today 2002; 7(1):5-7. doi: 10.1016/s1359-6446(01)02082-7 [Crossref] [ Google Scholar]
- Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv 2003; 3(2):90-105. doi: 10.1124/mi.3.2.90 [Crossref] [ Google Scholar]
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev 2007; 59(6):454-77. doi: 10.1016/j.addr.2007.04.011 [Crossref] [ Google Scholar]
- Patel M, Souto EB, Singh KK. Advances in brain drug targeting and delivery: limitations and challenges of solid lipid nanoparticles. Expert Opin Drug Deliv 2013; 10(7):889-905. doi: 10.1517/17425247.2013.784742 [Crossref] [ Google Scholar]
- Pardridge WM. Crossing the blood-brain barrier: are we getting it right?. Drug Discov Today 2001; 6(1):1-2. doi: 10.1016/s1359-6446(00)01583-x [Crossref] [ Google Scholar]
- Ricci M, Blasi P, Giovagnoli S, Rossi C. Delivering drugs to the central nervous system: a medicinal chemistry or a pharmaceutical technology issue?. Curr Med Chem 2006; 13(15):1757-75. doi: 10.2174/092986706777452461 [Crossref] [ Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57(2):178-201. doi: 10.1016/j.neuron.2008.01.003 [Crossref] [ Google Scholar]
- Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res 2007; 24(9):1745-58. doi: 10.1007/s11095-007-9374-5 [Crossref] [ Google Scholar]
- Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev 2001; 46(1-3):247-79. doi: 10.1016/s0169-409x(00)00139-3 [Crossref] [ Google Scholar]
- Smith MW, Gumbleton M. Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. J Drug Target 2006; 14(4):191-214. doi: 10.1080/10611860600650086 [Crossref] [ Google Scholar]
- Tsuji A, Tamai I. Blood-brain barrier function of P-glycoprotein. Adv Drug Deliv Rev 1997; 25(2-3):287-98. doi: 10.1016/s0169-409x(97)00504-8 [Crossref] [ Google Scholar]
- Schinkel AH. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev 1999; 36(2-3):179-94. doi: 10.1016/s0169-409x(98)00085-4 [Crossref] [ Google Scholar]
- Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol 2000; 2(1):45-59. doi: 10.1093/neuonc/2.1.45 [Crossref] [ Google Scholar]
- Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res 1999; 58(3):312-28. doi: 10.1006/mvre.1999.2188 [Crossref] [ Google Scholar]
- Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering?. J Struct Biol 2001; 136(3):239-55. doi: 10.1006/jsbi.2002.4441 [Crossref] [ Google Scholar]
- Brigger I, Morizet J, Aubert G, Chacun H, Terrier-Lacombe MJ, Couvreur P. Poly(ethylene glycol)-coated hexadecylcyanoacrylate nanospheres display a combined effect for brain tumor targeting. J Pharmacol Exp Ther 2002; 303(3):928-36. doi: 10.1124/jpet.102.039669 [Crossref] [ Google Scholar]
- Plate KH, Scholz A, Dumont DJ. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol 2012; 124(6):763-75. doi: 10.1007/s00401-012-1066-5 [Crossref] [ Google Scholar]
- Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles?. Drug Discov Today 2008; 13(23-24):1099-106. doi: 10.1016/j.drudis.2008.09.005 [Crossref] [ Google Scholar]
- van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 2015; 19:1-12. doi: 10.1016/j.drup.2015.02.002 [Crossref] [ Google Scholar]
- Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell 2017; 31(3):326-41. doi: 10.1016/j.ccell.2017.02.009 [Crossref] [ Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19(11):1423-37. doi: 10.1038/nm.3394 [Crossref] [ Google Scholar]
- Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT. Medulloblastoma Genotype dictates blood brain barrier phenotype. Cancer Cell 2016; 29(4):508-22. doi: 10.1016/j.ccell.2016.03.002 [Crossref] [ Google Scholar]
- Sampson JH, Maus MV, June CH. Immunotherapy for brain tumors. J Clin Oncol 2017; 35(21):2450-6. doi: 10.1200/jco.2017.72.8089 [Crossref] [ Google Scholar]
- Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 2018;33(5):874-89.e7. 10.1016/j.ccell.2018.03.020.
- Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015; 36(10):569-77. doi: 10.1016/j.it.2015.08.006 [Crossref] [ Google Scholar]
- Tivnan A, Heilinger T, Lavelle EC, Prehn JH. Advances in immunotherapy for the treatment of glioblastoma. J Neurooncol 2017; 131(1):1-9. doi: 10.1007/s11060-016-2299-2 [Crossref] [ Google Scholar]
- Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018; 24(6):724-30. doi: 10.1038/s41591-018-0040-8 [Crossref] [ Google Scholar]
- Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia 2002; 40(2):252-9. doi: 10.1002/glia.10147 [Crossref] [ Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330(6005):841-5. doi: 10.1126/science.1194637 [Crossref] [ Google Scholar]
- Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016; 19(1):20-7. doi: 10.1038/nn.4185 [Crossref] [ Google Scholar]
- Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Front Surg 2016; 3:11. doi: 10.3389/fsurg.2016.00011 [Crossref] [ Google Scholar]
- Bowman RL, Joyce JA. Therapeutic targeting of tumor-associated macrophages and microglia in glioblastoma. Immunotherapy 2014; 6(6):663-6. doi: 10.2217/imt.14.48 [Crossref] [ Google Scholar]
- Weller M, Roth P, Preusser M, Wick W, Reardon DA, Platten M. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol 2017; 13(6):363-74. doi: 10.1038/nrneurol.2017.64 [Crossref] [ Google Scholar]
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A 2013; 110(47):19059-64. doi: 10.1073/pnas.1318022110 [Crossref] [ Google Scholar]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010; 7(11):653-64. doi: 10.1038/nrclinonc.2010.139 [Crossref] [ Google Scholar]
- Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 2018; 4(4):292-319. doi: 10.1016/j.trecan.2018.02.005 [Crossref] [ Google Scholar]
- Chin L, Xia Y, Discher DE, Janmey PA. Mechanotransduction in cancer. Curr Opin Chem Eng 2016; 11:77-84. doi: 10.1016/j.coche.2016.01.011 [Crossref] [ Google Scholar]
- Jain RK, Wei J. Dynamics of drug transport in solid tumors-distributed parameter model. J Bioeng 1977; 1(4):313-29. [ Google Scholar]
- Karls MS, Rush BD, Wilkinson KF, Vidmar TJ, Burton PS, Ruwart MJ. Desolvation energy: a major determinant of absorption, but not clearance, of peptides in rats. Pharm Res 1991; 8(12):1477-81. doi: 10.1023/a:1015882030289 [Crossref] [ Google Scholar]
- Pauletti GM, Gangwar S, Siahaan TJ, Aubé J, Borchardt RT. Improvement of oral peptide bioavailability: peptidomimetics and prodrug strategies. Adv Drug Deliv Rev 1997; 27(2-3):235-56. doi: 10.1016/s0169-409x(97)00045-8 [Crossref] [ Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 2016; 1(5):16014. doi: 10.1038/natrevmats.2016.14 [Crossref] [ Google Scholar]
- Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 2017; 17(1):20-37. doi: 10.1038/nrc.2016.108 [Crossref] [ Google Scholar]
- Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 1968; 26(1):62-9. doi: 10.1016/0021-9797(68)90272-5 [Crossref] [ Google Scholar]
- Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know?. Adv Drug Deliv Rev 2014; 71:2-14. doi: 10.1016/j.addr.2013.08.008 [Crossref] [ Google Scholar]
- Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev 2012; 64(7):701-5. doi: 10.1016/j.addr.2011.12.006 [Crossref] [ Google Scholar]
- Gasco MR. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. Google Patents; 1993.
- Gasco MR. Lipid nanoparticles: perspectives and challenges. Adv Drug Deliv Rev 2007; 59(6):377-8. doi: 10.1016/j.addr.2007.05.004 [Crossref] [ Google Scholar]
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull 2015; 5(3):305-13. doi: 10.15171/apb.2015.043 [Crossref] [ Google Scholar]
- Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 2002; 242(1-2):121-8. doi: 10.1016/s0378-5173(02)00180-1 [Crossref] [ Google Scholar]
- Feng L, Mumper RJ. A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett 2013; 334(2):157-75. doi: 10.1016/j.canlet.2012.07.006 [Crossref] [ Google Scholar]
- Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs?. Eur J Pharm Sci 2012; 47(1):139-51. doi: 10.1016/j.ejps.2012.05.010 [Crossref] [ Google Scholar]
- Alam T, Pandit J, Vohora D, Aqil M, Ali A, Sultana Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv 2015; 12(2):181-94. doi: 10.1517/17425247.2014.945416 [Crossref] [ Google Scholar]
- Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano 2009; 3(1):16-20. doi: 10.1021/nn900002m [Crossref] [ Google Scholar]
- Mi L, Zhang X, Yang W, Wang L, Huang Q, Fan C. Artificial nano-bio-complexes: effects of nanomaterials on biomolecular reactions and applications in biosensing and detection. J Nanosci Nanotechnol 2009; 9(4):2247-55. doi: 10.1166/jnn.2009.se25 [Crossref] [ Google Scholar]
- Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm 2009; 71(2):251-6. doi: 10.1016/j.ejpb.2008.08.021 [Crossref] [ Google Scholar]
- Chang J, Paillard A, Passirani C, Morille M, Benoit JP, Betbeder D. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res 2012; 29(6):1495-505. doi: 10.1007/s11095-011-0624-1 [Crossref] [ Google Scholar]
- Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target 2011; 19(2):125-32. doi: 10.3109/10611861003734001 [Crossref] [ Google Scholar]
- Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol Immunother 2007; 56(8):1215-23. doi: 10.1007/s00262-006-0273-0 [Crossref] [ Google Scholar]
- Zhang JZ. Biomedical applications of shape-controlled plasmonic nanostructures: a case study of hollow gold nanospheres for photothermal ablation therapy of cancer. J Phys Chem Lett 2010; 1(4):686-95. doi: 10.1021/jz900366c [Crossref] [ Google Scholar]
- Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine 2011; 7(1):69-79. doi: 10.1016/j.nano.2010.06.010 [Crossref] [ Google Scholar]
- Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012; 33(32):8167-76. doi: 10.1016/j.biomaterials.2012.07.046 [Crossref] [ Google Scholar]
- Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Control Release 2007; 122(1):1-9. doi: 10.1016/j.jconrel.2007.05.022 [Crossref] [ Google Scholar]
- Assa-Munt N, Jia X, Laakkonen P, Ruoslahti E. Solution structures and integrin binding activities of an RGD peptide with two isomers. Biochemistry 2001; 40(8):2373-8. doi: 10.1021/bi002101f [Crossref] [ Google Scholar]
- Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswal S. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials 2009; 30(36):6912-9. doi: 10.1016/j.biomaterials.2009.08.045 [Crossref] [ Google Scholar]
- Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res 2007; 16(8):351-9. doi: 10.3727/000000006783980946 [Crossref] [ Google Scholar]
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 2009; 6(3):659-68. doi: 10.1021/mp900015y [Crossref] [ Google Scholar]
- Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 2008; 4(3):372-9. doi: 10.1002/smll.200700784 [Crossref] [ Google Scholar]
- Hashizume R, Frey WH II. Intranasal delivery–a new therapeutic approach for brain tumors. US Neurol 2008; 4(2):43-4. [ Google Scholar]
- Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 2009; 379(1):146-57. doi: 10.1016/j.ijpharm.2009.06.019 [Crossref] [ Google Scholar]